Journal of
eISSN: 2373-4426


Case Report Volume 2 Issue 1
1Department of Pediatrics, MacKay Children's Hospital, Taiwan
2MacKay Junior College of Medicine Nursing and Management, Taiwan
Correspondence: Ching-Ying Huang, Department of Pediatrics, MacKay Childrens Hospital, 92, Section 2, Chung-Shan North Road, Taipei, Taiwa, Tel +886-2-2543-3535, Fax +886-2-2543-3642
Received: December 15, 2014 | Published: January 14, 2015
Citation: Huang CY, NC Chiu (2015) Chylothorax Secondary to Late-Onset Congenital Diaphragmatic Hernia. J Pediatr Neonatal Care 2(1): 00055. DOI: 10.15406/jpnc.2015.02.00055
Late-onset congenital diaphragmatic hernia and chylothorax are rarely associated. We reported a 3-month-old male infant who presented with acute respiratory and gastrointestinal symptoms. Computed tomography of his chest showed features of diaphragmatic hernia with pleural effusion. Biochemistry charterers of pleural effusion identified chylothorax. The chylous effusion was drained and the diaphragmatic hernia was repaired by laparoscopic surgery. After surgery, the lung was fully expanded. Chylothorax was cured. In this case, lymphatic obstruction caused by the weight of the herniated bowel loops or by compression of lung may result in increased lymphatic pressure and contribute to the leakage of chyle into the pleural cavity.
Keywords: chylothorax, chylous effusion, diaphragmatic hernia, late onset, infant,child
Congenital diaphragmatic hernia (CDH) is a structural diaphragmatic defect that usually presents during the first few hours after birth with severe respiratory distress. However, delayed herniation of the abdominal contents through a CDH may occur beyond neonatal period. In contrast to the high mortality and morbidity rates for neonatal CDH, the prognosis for late-presenting CDH is usually favorable if diagnosed earlier. However, unlike in neonates, the spectrum of manifestations of late-presenting CDH is quite broad and nonspecific. The patients may present with acute or chronic respiratory, gastrointestinal symptoms or be completely asymptomatic.1 The variety of clinical manifestations for late-onset CDH makes the early diagnosis challenging. Chylothorax, the accumulation of chyle in the pleural space, is a relatively rare cause of pleural effusion in children after neonatal period.2 Late-presenting CDH and chylothorax are rarely associated. We reported a 3-month-old infant of late-presenting CDH with development of chylous effusion.
A 3-month-old male infant brought to our hospital with a one-day history of poor appetite and activity. Gestational history was uneventful with birth weight 3520grams and normal Apgar scores. He was healthy and had good weight gain before admission. The physical examination revealed severe respiratory distress, tachycardia, and acute ill looking. He had grunting, nasal flaring, and subcostal retraction. Air entry was reduced on the left side of the chest. His abdomen was flat with hypoactive bowel sounds. Chest radiograph showed opacification of left hemithorax with a moderate shift of mediastinum to right (Figure 1). He was admitted to the pediatric intensive care unit.
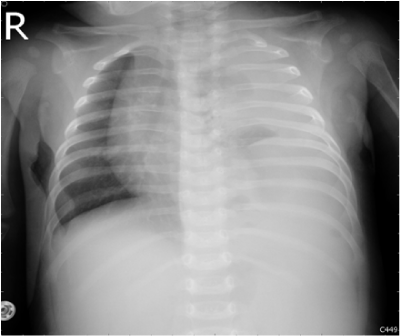
Figure 1 Initial chest x-ray showed left-sided opacification and a moderate shift of the mediastinum to the right.
The hematologic and biochemical investigations were unremarkable except for leukocytosis (18, 800cells/μL). Clear yellowish fluid was drained from chest tube and the pleural fluid turned to milky white on the next day. Pleural fluid analysis revealed pH 7.5, LDH 116U/L, protein 20.9g/L, glucose 362mg/dL, total cholesterol 8 mg/dL, triglyceride 391mg/dL, and predominance of lymphocytes, which of them were all compatible with chylous effusion. A computed tomography of the chest and abdomen showed left pleural effusion, lung atelectasis and retention of bowel loops in the anterior aspect of left hemi-thorax (Figure 2). The finding was consistent with diaphragmatic hernia.
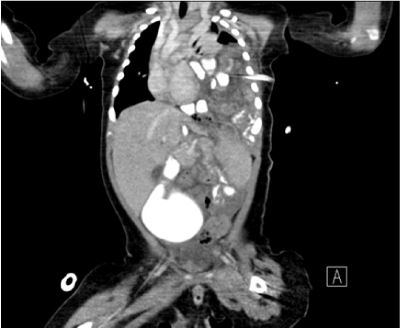
Figure 2 Chest CT demonstrated left diaphragmatic hernia and bowel loops, which contained barium contrast, in left thorax with pleural effusion and lung atelectasis.
Surgical reduction was performed by laparoscope 2days later. During laparotomy, we found transverse colon and small bowel loops were herniated to left pleural cavity. A left posterior-lateral defect of diaphragm, measuring 3.5 × 4cm2, was repaired with absorbable mesh and fixed by screws. There was no perforation of bowel or thoracic duct. On the day following surgery, the patient was able to ingest formula, and was discharged 5days postoperatively in good condition. The follow-up chest radiograph showed full expansion of lung and normal position of diaphragm.
CDH is estimated to occur in 1 of 2,000-4,000 births.3 It is an embryonic malformation of the diaphragm occurring around the eight to ninth week of gestation and usually presents with respiratory distress soon after birth.4 However, some patients develop symptoms after the neonatal period. Late-presenting diaphragmatic hernia accounts 2.6-20% for all CDH and may first manifest at any age.5,6 The presentations of late-presenting CDH have wide variety including, in addition to respiratory distress, gastrointestinal obstruction, growth retardation, perforations or strangulations of intrathoracic hollow viscera, rupture of a herniated spleen, airway infections or recurrent pneumonias, urinary tract obstruction due to herniation of the ureter, intrathoracic appendicitis and other rare presentations.7,8 Chest radiography is usually the first diagnostic modality upon which further management is based. It is assumed that the presence of intrathoracic translucent structures along with mediastinal shift is the most characteristic radiological feature of late-presenting CDH, but only when hernia contains stomach or bowel. Misinterpretation of initial radiographic findings occurred in more than 25% of the children and nearly 50% of the children require additional imaging studies to achieve a final diagnose.9 Our patient is a 3-month-old infant presenting with acute respiratory and gastrointestinal symptoms. Chest x-ray revealed opacification of left hemithorax with shifted mediastina. Further computed tomography made a final diagnosis of CDH. Under prompt detection, the patient’s herniation of bowel loops was reduced and the affecting lung was fully expanded after surgery. His follow-up condition is well 3months later.
The association of chylothorax characterizes this patient. From review of the literature, we did not found previously reported case of an infant with late-presenting CDH causing chylous effusion. Chylothorax is a pleural collection of fluid formed by the escape of chyle from the thoracic duct or lymphatics into the thoracic cavity. It is the most common form of pleural effusion in neonate but is a rare cause of pleural effusion in infants and children.2,10 Chyle is a non-inflammatory, alkaline and bacteriostatic fluid composed mainly of fat, cholesterol, electrolytes, proteins, glucose, and abundant lymphocytes. The protein content of chyle is usually more than 3g/L, and the electrolyte composition is similar to that of serum. The cells are primarily T lymphocytes.11 Measuring the triglyceride and cholesterol levels in the pleural fluid is the best way to diagnose chylothorax. If the triglyceride is above 110mg/dL and the ratio of the pleural fluid to serum cholesterol is less than 1.0, the diagnosis is established.12 In our case, the milky fluid from the patient showed marked triglyceride elevation and low ratio of pleural fluid to serum cholesterol, which were compatible with chylothorax.
In normal physiology, the gut lymphatics carry converted long-chain triglycerides from the intestine to the vascular system via the thoracic collecting duct, which transports 1.5 to 2.5liters of chyle daily in children.2 As a result, in cases of acute lymphatic obstruction, rapid accumulation of chyle can occur. In children, development of chylothorax is more commonly associated with cardiothoracic surgery with an incidence of 65-90%.13,14 Other important causes in children include congenital lymphatic malformations, associated with congenital anomaly, non-iatrogenic trauma, and tumors.15 In our patient, we postulate that the herniation of intestinal loops in the thorax had led obstruction of lymphatic flow from the weight of the bowel loops or compression of lung. The increasing lymphatic pressure contributed to the leakage of chyle, which eventually accumulated in the pleural cavity.
The optimal management of chylothorax depends on its underlying etiology. Imaging studies such as computed tomography, lymphangiography, and lymphoscintigraphy can be helpful to determine the cause of the chylothorax.2 In our case, thoracic duct injury by chest tube was considered but less likely since this procedure was echo-guided and the operation performed later did not find evidence of thoracic duct damage. The patient’s chylothorax also totally resolved after the surgery. Hence, the cause of the chylous effusion could be confidently attributed to herniation of bowel loops, which resulted in temporary lymphatic obstruction.
We would like to acknowledge Dr. Nien-Lu, Wang for his assistance with performing the surgery.
Author declares there are no conflicts of interest.
None.

©2015 Huang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.