Journal of
eISSN: 2373-4426


Research Article Volume 6 Issue 2
Neonatal Unit, Department of Paediatrics, National Hospital Abuja, Nigeria
Correspondence: Lamidi I Audu, Neonatal Unit, Department of Paediatrics, National Hospital Abuja, Nigeria, Tel 2350000000000
Received: December 25, 2016 | Published: February 13, 2017
Citation: Audu LI, Mairami AB, Otuneye AT, Adeleye QAQ (2017) Can Post Exchange Blood Transfusion Haematocrit Be Predicted in Neonates? J Pediatr Neonatal Care 6(2): 00241. DOI: 10.15406/jpnc.2017.06.00241
Background: Double volume exchange transfusion (DVET) primarily treats severe hyperbilirubinaemia; it also corrects coexisting anaemia when donor blood haematocrit is appropriately adjusted. In resource poor countries, blood for exchange transfusion (ET) is limited to fresh whole blood, the haematocrit of which is rarely documented. In Nigeria, top up transfusion is given to correct anticipated post ET anaemia. This practice is a potential cause of hypervolemia, polycythaemia and hyperviscosity.
Objectives: We set out to determine the predictability of post ET haematocrit as this could help identify jaundiced babies that will benefit from post- exchange top up transfusion.
Subjects and Methods: Severely jaundiced newborn babies exchanged using the hospital standard protocol were prospectively studied. Blood was drawn from baby before commencement and within one hour after completing the procedure for pre and post ET haematocrit respectively. Donor blood haematocrit was also documented. Post ET haematocrit was calculated on the assumption that DVET resulted in 90% replacement of baby’s blood. Bland-Altman analysis was done to estimate the level of agreement between calculated and observed post ET haematocrit.
Results: Forty out of 56 babies (30 term) met the inclusion criteria and were analyzed. Mean donor haematocrit was 41.2(4.3)%. This was not significantly different from the pre ET haematocrit (39.9(9.0)% P=.366. Pre ET haematocrit was also similar to post ET haematocrit {39.1(3.7)% P=.107}. Estimated haematocrit {40.5(4.1)%} was similar to post ET observed haematocrit. There was a significant level of agreement between observed and estimated haematocrit. Mean difference =-0.887±3.44 (95% CI=-7.8, 6.0: P=.422).
Conclusion and Recommendation: It is concluded that post ET haematocrit can be estimated with a reasonable level of accuracy provided the donor blood haematocrit is known. Jaundiced babies should be selectively identified for post exchange transfusion.
Key words: neonates, exchange transfusion, haematocrit, jaundice
DVET, double volume exchange transfusion; ET, exchange transfusion; Hd, donor haematocrit; He, expected/estimated haematocrit; Ho, observed haematocrit; Hp, pre et haematocrit; FBC/DIFF, full blood count and differentials; U/E, urea and electrolytes. RR, respiratory rate; HR, heart rate
Double volume exchange transfusion (ET) removes a substantial amount of bilirubin and is therefore primarily targeted at treating severe and extreme hyperbilirubinaemia to avert the danger of bilirubin encephalopathy. It also alters the haematocrit level to an extent predominantly dependent on the haematocrit of the blood used for this procedure.1 It is estimated that at the conclusion of a double volume exchange transfusion, about 90% of baby’s blood would have been exchanged. While ET is rarely done in most developed countries, it is a common life-saving therapeutic procedure in Nigeria where severe neonatal hyperbilirubinaemia is frequently encountered.2−5
Although there is no evidence in support of routine post exchange top-up blood transfusion, this is a common practice in most parts of Nigeria. An electronic survey of randomly selected Paediatric practitioners from different parts of the country revealed that babies receive post ET top up transfusion for various reasons the most common of which is to compensate for blood spills during the procedure. The volume of blood for top up transfusion was also empirically based on perceived volume of blood spill. These findings suggested an informal consensus for a clinical practice which could result in a positive volume balance (the volume transfused being in excess of blood withdrawn from the baby), with the potential risk of significant fluid overload, polycythaemia and hyperviscosity. This practice has however rarely been reported in the literature. In a retrospective review of neonatal transfusions over a period of 12months at a teaching hospital in South West Nigeria, Ogunlesi et al.,6 reported that 37 of 77 (49.3%) babies, who had exchange transfusion for severe neonatal jaundice, also received post exchange top up transfusion. The indications for these top up transfusions were however not stated.
A decrease in haematocrit observed after double volume exchange transfusion was attributed to ongoing haemolysis and inadequate mixing of donor blood during ET 7 or technical errors during the procedure.8 The clinical significance of this post ET drop in haematocrit was not stated and top up transfusion was not done. Contrary to the findings from the studies above,6−8 Ballot et al.,9 from South Africa did not observe any significant change in haematocrit following double volume ET.
Donor blood haematocrit is the major determinant of post ET haematocrit. Ghaemi et al.,1 demonstrated a significantly higher haemoglobin level in patients exchanged with ‘dry packed cells’ (PCV>70%) compared with babies who had ET with either regular packed cells or compatible fresh whole blood. No explanation was given for using blood with haematocrit of over 70%. Sharma et al.,7 noted a20% drop in haematocrit, post ET in the first 4 babies who had ET with blood reconstituted to a haematocrit of 45% in their study. Raising the haematocrit of reconstituted blood to 50% in exchange transfusions for subsequent babies resulted in significantly improved post ET haematocrit.
In Nigeria, reconstituted blood with predetermined haematocrit is not available in most blood banks. Exchange transfusion is therefore traditionally done with compatible fresh whole blood and donor blood haematocrit is not routinely requested. This may be the basis for the ‘informal consensus’ on post ET top up transfusion. It is therefore imperative to determine the post ET haematocrit in our neonates to provide evidence to justify the need or otherwise, for post ET top up transfusion. Furthermore, it is theoretically possible to calculate expected haematocrit following double volume exchange transfusion, provided donor haematocrit is known. If this estimate agrees reasonably with the laboratory determined haematocrit, early identification of babies for selective post ET top up transfusion can be reliably doneand this will replace the current practice of routine post ET top up.
The objectives of this study were therefore to determine post ET haematocrit as well as investigate its predictability in babies with severe neonatal jaundice. We hypothesized that (1): there was no significant difference between Pre and post ET haematocrit in severely jaundiced babies and 2: calculated (expected) and laboratory (observed) post ET haematocrit were not significantly different from each other.
Subjects
Newborn babies aged 0-14days with gestational age of 32-42weeks, admitted into the neonatal unit of the department of Paediatrics, National Hospital Abuja with pathologic jaundice, who had double volume exchange transfusion (DVET) were prospectively enrolled into the study after obtaining parental consent. If the blood used for EBT was more or less than the calculated volume (2 x 85ml/ Kg for term babies and 2 x 90ml/kg for preterm babies), the patient was excluded, as was the case with those whose parents or guardian refused to participate in the study.
Indication for EBT
All jaundiced babies who met the inclusion criteria were consecutively recruited into the study from August 2015 to October 2016.Consent was sought from parents/guardian for EBT following departmental protocol for this procedure, but for the purpose of this study, additional information on study objective was provided. Ethical approval was obtained from the National hospital Ethics committee.
For each baby, relevant medical and demographic information were documented. These included gestational age, sex, age at admission, blood group of parents and baby, duration of membrane rupture and maternal peripartum fever. In addition to serum bilirubin determination other investigations carried out included pre and post ET haematocrit and donor blood haematocrit. FBC/DIFF, U/E and Creatinine, Coombs test and blood cultures were done for suspected sepsis, as part of the routine investigations in the management of jaundiced babies.
Procedure: Exchange transfusion was carried with fresh whole blood (<48hours) compatible with mother and baby, usually O+ (O- if baby was blood group O-). The method used for exchange blood transfusion was standardized for all babies in the study; the push–pull method via the umbilical vein with the catheter attached to a three-way plastic device. The volume of blood used was 85ml/kg for term babies and 90mls/kg for preterm babies. Blood was drawn from baby and replaced with donor blood in aliquots of 5ml/kg and this pull-push cycle which lasted for 2-3minutes, was repeated until the calculated volume of blood had been exchanged. Donor blood bag was intermittently gently agitated to avoid sedimentation of red cells. Precautions were taken to ensure asepsis, minimize blood spill and maintain a warm environment throughout the period of exchange. Oral feeding was suspended for 2hours before, to 2hours after the procedure. At the end of every 10 cycles, 0.5-1ml of calcium gluconate was given. The assisting Nurse monitored and documented the vital signs (RR, HR, temperature) at the end of every cycle. Pre ET sample (1ml) for haematocrit was taken from the first aliquot of blood withdrawn from baby (and immediately taken to the laboratory) while the same volume of blood was drawn from a peripheral vein for post ET haematocrit within one hour of completing the procedure. One ml of donor blood was withdrawn from the bag for donor haematocrit. Both samples were also taken to the laboratory for haematocrit determination.
Data analysis
Data obtained were checked for completeness and entered into a spreadsheet. Statistical analysis was done using SPSS version 21.General bio-demographic data were presented in frequency tables, mean and standard deviations were computed for haematologic variables and paired t-test was used to analyze the relationship between pre and post ET haematocrit as well as pre and post ET serum bilirubin.
Expected post ET haematocrit was calculated for each baby on the assumption that double volume exchange transfusion (DVET) would result in 90% exchange of baby’s blood12:
He= (% of blood exchanged x Hd + % unexchanged blood x Hp)/100.13
He: Expected Haematocrit; Hd: Donor Haematocrit and Hp: Baby’s Pre EBT Haematocrit
The Bland- Altman analysis14 was used to test the level of agreement between calculated (expected) and observed (laboratory) haematocrit (He and Ho). The plot relates the difference in He and Ho with the mean of He and Ho.
Fifty six babies had DVET and were recruited into the study; 10 were excluded from analysis because the volume of blood used was either less or more than the volume calculated while in 6, pre ET or donor sample haematocrit was not available. Forty babies were therefore analyzed. These consisted of 25 males and 15 females; 30 term and 10 preterm babies. The cause of jaundice was known in 29 (72.5%) babies out of whom haemolytic causes accounted for 19(65.5%). Mean donor haematocrit was 41.2 (4.3)% with a range of 34.0% to 53.0%. Table 2 shows the haematologic parameters. Pre EBT haematocrit 39.8(9.0)% was similar to the post ET haematocrit 39.1(3.7)%, P=.983. Disaggregated analysis also showed similar relationship in the haemolytic and non-haemolytic jaundice groups {(40.1(3.7)% vs 41.2(3.9)% and 40.6(3.8)% vs 39.6(4.5)% respectively. P=.108 and .543}. Donor blood haematocrit was not significantly different from pre ET haematocrit; P=.366.
Variable |
Frequency(%)/mean(SD) |
||
Sex: n=40 |
|||
M |
25(62.5) |
||
F |
15(37.5) |
||
Gestation: n=40 |
|||
Term |
30(75) |
||
Preterm |
10(25) |
||
Type of jaundice: n=40 |
|||
Haemolytic |
19(47.5) |
||
Non haemolytic |
10(25.0) |
||
Others |
11(27.5) |
||
Age at presentation(hrs) |
111.6(54.6) |
||
Volume of blood for ET(mls) |
369.9(160.1) |
||
Donor haematocrit (%) |
41.2(4.3) |
||
Pre-ET haematocrit (%) |
39.8(9.6) |
||
Pre-ET SB(mg/dl) |
26.6(10.4) |
||
Table 1 General Characteristics
|
PreET |
PostET |
df |
t |
P |
|
Haematocrit |
39.8 (9.6) |
39.1 (3.7) |
39 |
-1.648 |
0.107 |
|
Serum bilirubin |
26.6 (10.4) |
12.0 (6.5) |
39 |
11.764 |
0 |
|
Haematocrit |
Pre ET |
Donor |
df |
t |
P |
|
|
39.8(9.6) |
41.3(4.3) |
38 |
0.914 |
0.366 |
|
Table 2 Relationship between donor, Pre ET and Post ET Haematocrit and serum bilirubin
Footnote: ET: Exchange Transfusion; SB: Serum Bilirubin
|
Ho % |
He% |
df |
t |
P |
All babies |
39.1(3.7) |
40.5(4.1) |
39 |
-1.648 |
0.107 |
Haemolytic |
40.1(3.70 |
41.2(3.9) |
18 |
-1.691 |
0.108 |
Nonhaemolytic |
40.6(3.8) |
39.9(4.6) |
9 |
0.633 |
0.543 |
Others |
37.8(3.2) |
39.7(3.8) |
10 |
-1.483 |
0.169 |
Table 3 Relationship between observed haematocrit (Ho) and expected haematocrit (He)
Calculated (expected) haematocrit 40.5(4.1)% was not significantly different from the observed post ET haematocrit 39.1(3.7)%, P=.107. The degree of agreement was assessed using the Bland-Altman method which plots the difference in means of the observed and expected haematocrit (Ho - He) against the means of the two haematocrit values; (Ho + He/2). The mean difference of expected haematocrit (He) and observed post- exchange transfusion haematocrit (Ho) was -0.8875 ± 3.4496. (95% C I = 6.0, -7.8). Figure 1 shows the Bland Altman’s plot.
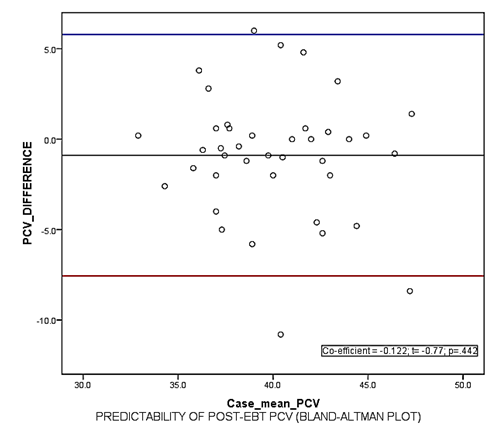
Figure 1 Bland-Altman Plot for level of agreement between expected and observed post EBT haematocrit/PCV (packed cell volume).
There was a 54.9% reduction in the serum bilirubin level from 26.6(10.4)mg/dl to 12.0(6.5)mg/dl P=.000.
This study examined the haematocrit in babies who underwent double volume exchange transfusion for severe hyperbilirubinaemia with compatible fresh whole blood. Although post ET haematocrit is usually normal, this expectation is based on the assumption that the donor blood for ET is adjusted to the desired haematocrit level using reconstituted blood (Packed red cells + fresh frozen plasma). In clinical practice we are restricted to the use of fresh whole blood, the haematocrit of donor blood is not routinely requested and this forms the basis for routine post EBT top up transfusion.
Our study has shown a mean (SD) post ET haematocrit of 39.1(3.7)%. This did not differ significantly from the pre ET haematocrit 39.6(9.6)%. Both Pre and post ET haematocrit in our babies are lower than the mean haematocrit reported by Buseri et al.,11 for healthy Nigeria babies in the first week of life. The mean age of the babies in this study was 4.7(2.3) days. Our babies were therefore mildly anaemic before they were exchanged and this persisted after the double volume exchange transfusion. There was no significant difference in haematocrit levels between haemolytic and non haemolytic groups. It is noteworthy that the mean donor blood haematocrit in our study was 41.2(4.3)% and this was not significantly different from the pre ET haematocrit of the babies. It therefore implies that our jaundiced babies were anaemic before the exchange transfusion and the donor blood haematocrit was not sufficiently high to cause a significant increase in the post-exchange haematocrit. This underscores the need for improvement in our blood banking services with emphasis on the use of reconstituted blood with predetermined haematocrit to meet the specific need of the severely jaundiced baby with coexisting severe anemia who requires exchange transfusion.
Ibekwe et al.,3 retrospectively reviewed the records of 40 babies who had double volume ET for neonatal jaundice from the Eastern part of Nigeria and documented a mean pre ET haematocrit of 35.92(7.71), which was lower than what we got in this study. The post EBT haematocrit was however not documented in their study. The Pre ET haematocrit in babies in a South African study was 39.6% and in agreement with our findings, was not significantly different from the post-exchange haematocrit 42%.9 In contrast to our finding however, Akrem 8 reported a much higher mean pre EBT haematocrit of 49.62(9.6)%. Although a drop in haematocrit post EBT was said to have been observed in some of their babies, the mean post-exchange haematocrit was not stated. While we noted that post ET haematocrit in our patients was low, it was clearly above the recommended transfusion threshold of 30-35% for babies in the first week of life.15,16 Such babies should however be closely monitored and offered blood transfusion only when indicated.
Based on the assumption that double volume ET replaces about 90% of baby’s blood with donor blood, we estimated the post EBT haematocrit for all babies using the pre EBT haematocrit and donor haematocrit as earlier described. The mean estimated (expected) haematocrit 40.5(4.1)% was similar to the haematocrit obtained from the laboratory (observed) 39.1(3.7)%, P=.107. This finding was similar for both haemolytic and non haemolytic groups. The validity of this finding was further confirmed by subjecting the values of estimated and observed haematocrit to Bland-Altman’s analysis to determine the level of agreement between them. This showed a significant level of agreement (Figure 1). The mean difference between expected haematocrit (He) and observed post- exchange transfusion haematocrit (Ho) was -0.8875 ±3.4496 (95% C I = -7.8-6.0). This implies that in 95% of the babies, the difference between He and Ho falls within a range of -7.8% to +6%. It therefore implies that the post ET haematocrit can be estimated within this limit of accuracy before the procedure is commenced and the result therefore used to identify babies who will benefit from post ET top up transfusion. We believe this is of immediate relevance to our clinical practice.
Our assumption was that in all babies, double volume exchange transfusion would uniformly result in 90% replacement of baby’s blood. In practice this may not be the case, the level of replacement varies from 80% to 90%resulting in some level of variability in individual observed post exchange haematocrit. Furthermore, a larger sample size will most probably result in a higher level of agreement than we got in the present study. The anticipated effect of ongoing haemolysis on post ET haematocrit was also not seen and this may also be due to the sample size which in the disaggregated analysis (haemolytic/non-haemolytic) may not be adequately powered to reveal the impact of haemolysis on the final haematocrit.
We conclude that post ET haematocrit does not differ from pre ET haematocrit and this is most probably due to the low haematocrit of donor blood. The study has also shown that within the limit of our assumption (that DVET results in 90% replacement of baby’s blood), post ET haematocrit can be estimated with a reasonable level of accuracy. We therefore recommend that for all babies undergoing ET for severe hyperbilirubinaemia in Nigeria, donor blood haematocrit should be determined and post ET haematocrit estimated to identify the baby that may have significantly low post ET haematocrit, for post-exchange top up transfusion. Routine top up transfusion after exchange transfusion for neonatal jaundice should therefore be discouraged. Upgrading our laboratories to a level with the capacity to provide reconstituted blood of predetermined haematocrit for exchange transfusion is ultimately desirable.
Post ET haematocrit is affected by donor blood haematocrit.
Post ET haematocrit can be accurately predicted and this can be used to identify babies for top up transfusion.
We appreciate the entire staff of the newborn unit who participated in the management of the patients used in this study.
Author declares there are no conflicts of interest.
None.

©2017 Audu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.