Journal of
eISSN: 2373-4426


Research Article Volume 5 Issue 7
1Department of Pediatrics, Apollo Hospital, India
2Pediatric Intensive Care Unit, Apollo Hospital, India
3Department of Pediatrics and Neonatology, W hospital by Pratiksha, India
4Professor and Head of the Department, Department of Pediatric Neurology, Apollo Hospital, India
Correspondence: Surender Kumar, Indraprastha Apollo Hospital, Sarita Vihar, New Delhi, India, Tel 9953570052
Received: June 14, 2016 | Published: December 1, 2016
Citation: Kumar S, Bakshi AS, Chakrabarti R, Kalra V (2016) Buccal Clonazepam versus Intravenous (I/V) Midazolam for Management of Acute Breakthrough Seizure –A Randomized Controlled Trial. J Pediatr Neonatal Care 5(7): 00211. DOI: 10.15406/jpnc.2016.05.00211
Objectives: To compare efficacy and safety of buccal Clonazepam with I/V Midazolam in acute seizure therapy.
Methods: A prospective, open label randomized controlled trial done on children with acute breakthrough seizure in known epileptics .200 breakthrough episodes were randomized to inject able midazolam (0.2mg/kg) or buccal clonazepam (0.01mg/kg) given on even days (IV Midazolam) and odd days (buccal Clonazepam).
Results: Ninety two (92.0%) seizures were controlled IV Midazolam and eighty six (86.0%) in buccal Clonazepam group. No statistical significant (p=0.175) was observed .Recurrence after initial control was 8.0% in IV Midazolam and 9.0% in buccal Clonazepam group with no statistical significant (p=0.800) .Vital monitoring parameters maintained in both groups .16.0% revealed adverse reactions 10 min post drug administration, 4.0% in the midazolam, and 12.0% in clonazepam group (p value=0.119). Adverse effects required no intervention.
Conclusions: Buccal clonazepam demonstrated equivalence in safety &efficacy to the I/V midazolam given in acute breakthrough seizure.
Keywords: breakthrough seizure, buccal clonazepam, domiciliary treatment, seizure, midazolam, epilepticus
Seizures are one of the commonest neurological symptoms seen in children. Recurrent seizures could ensue from epilepsy, febrile seizures, provoked seizures, drug inefficacy or with, or due to unknown or systemic illnesses.1 A breakthrough seizure is an epileptic seizure that occurs, despite the use of anticonvulsants that have otherwise successfully prevented seizures in the patient. Breakthrough seizures may be more dangerous than non-breakthrough seizures as they are less expected by the patient. Epileptics with a higher intensity of seizures are more likely to suffer from a breakthrough seizure. Rates of breakthrough seizures vary; studies have shown the rates of breakthrough seizures ranging from 11-37 %.2,3
BZD are the first line anticonvulsants for treating status epilepticus and acute repetitive seizures. They offer the most rapid cessation of seizure activity regardless of the etiology. They are easy to administer, effective, have a quick onset of action and cross the blood brain barrier early.4 Hence their role in the emergency management of acute seizures. Intermittent use of BZD is especially suitable for patients with clusters of repetitive seizures. BZDs are versatile drugs, have broad spectrum of activity and can be administered by several routes.5–7
The present study compared buccal clonazepam v/s IV midazolam as standard care of treatment for acute seizure. If safe and efficacious the therapy can be extrapolated to domiciliary setting.
200 acute breakthrough seizure episodes were randomized to either inj midazolam (0.2mg/kg) or buccal clonazepam (0.01mg/kg) respectively. 100 episodes in each group were studied. Randomization was done on the basis of even days (IV Midazolam) and odd days (buccal Clonazepam). Severe cardiopulmonary compromised patients were excluded from the study .Study design is shown in Figure 1.
Efficacy
Defined as cessation of all clinical seizure activity within five minutes of drug administration.
Treatment failure
Seizures do not get aborted within five minutes after treatment.
Recurrent seizure
Seizures which were controlled with midazolam or clonazepam but recurred within 10 minutes of drug administration.
Monitoring
Patients were monitored for vital parameters including heart rate, respiratory rate, blood pressure, saturation for ten minutes. The side effects of each drug were compared in different age groups with different semiology and etiology of seizures. We used Clonazepam (0.25/0.50/1.00mg) mouth dissolving dispersible clonazepam tablets.
Statistical analysis
Data was recorded on a predesigned proforma and it was managed on a Microsoft Excel spread sheet. Statistical analysis was performed using software between the 2 groups: IV Midazolam and Buccal clonazepam. Categorical variables were summarized by frequency .Proportions were compared by chi-square test or Fisher’s exact test (where expected frequency <5). Continuous variables having a normal distribution were summarized by mean and standard deviation; for others median was used .for continuous variables, student’s ‘t’ test , Mann Whitney U test or Wilcoxon signed rank sum test were applied as appropriate .P value of <0.05 was taken as significant .
The baseline characteristics of two drug groups with respect to age, sex, etiology of seizures, types of seizure, duration of seizure,EEG,neuroimaging were statistically comparable (<p value 0.05) as shown in Table 1, Figure 2 &3.
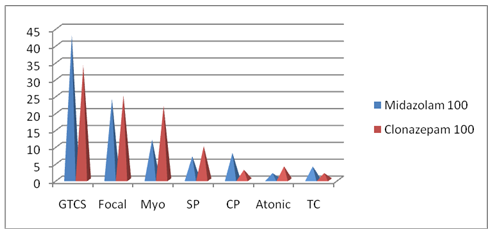
Figure 2 Seizure semiology in study groups (n=100).
GTCS, generalized tonic clonic; Myo, myoclonus; Sp, simple partial seizure; CP, complex partial seizure
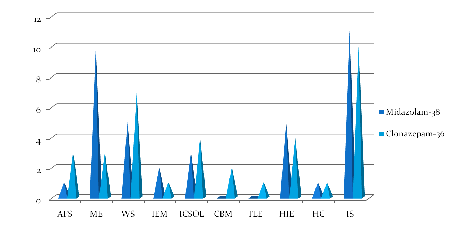
Figure 3 Comparison of etiology in both study groups (n=74).
AFS: Atypical Febrile Seizure; ME: Meningoencephalitis; WS: West’s Syndrome; IEM: Inborn Error of Metabolism; ICSOL: Intracranial Space Occupying Lesion; CBM: Congenital Brain Malformation; TLE: Temporal Lobe Epilepsy; HIE: Hypoxic Ischemic Encephalopathy; HC: Hypocalcemic Seizure; IS: Idiopathic Seizure
|
Midazolam |
Clonazepam |
P value |
Total Seizure Episodes |
100 |
100 |
- |
Total Children |
38 |
36 |
- |
Age,(years) |
5.23±4.85 |
4.35±3.65 |
0.708 |
Sex |
23(60.5%) |
24(66.7%) |
0.583 |
Family history |
2(2.0%) |
3(3.0%) |
0.670 |
Abnormal neurological examination |
15(39.5%) |
11(30.6%) |
0.422 |
Developmental delay |
9(23.7%) |
10(27.8%) |
0.687 |
Abnormal Neuroimaging |
21(55.2%) |
18(50.0%) |
0.629 |
EEG |
21(55.2%) |
24(66.6%) |
0.925 |
Duration of acute seizure (Minutes)prior to randomization |
3.39 |
3.56 |
0.559 |
Table 1 Comparison of baseline characteristics in IV midazolam and buccal clonazepam acute break through seizure
Ninety two (92.0%) seizure episodes were controlled in IV Midazolam group Vs eighty six (86.0%) in buccal Clonazepam group. The difference was not statistically significant (p=0.175). Recurrence after initial control was eight (8.0%) in IV Midazolam group and nine (9.0%) in buccal Clonazepam group, the difference was not statistically significant (p=0.800). Vital monitoring did not reveals any significant difference in both groups. Total of sixteen (16.0%) adverse reactions were observed after 10 min of drug administration in both groups, four (4.0%) in the IV midazolam group, and twelve (12.0%) in the buccal clonazepam group (p value=0.119). All adverse effects were transient, and No intervention was required.
Among the various routes of drug delivery, the oral route is the most preferred by the patient and the clinician .Oral administration of drugs have disadvantages such as hepatic first pass metabolism, enzymatic degradation within the GI tracts, that prohibits oral administration of drugs especially peptides and proteins. Consequently, other absorptive mucosae are considered sites for drug administration.8 Transmucosal routes of drug delivery (i.e., the mucosal linings of the nose, rectum, and vagina, ocular and oral cavity) offer potential systemic drug delivery. The advantages include possible bypass of first pass effect, avoidance of presystemic elimination within the GI tract, and, a better enzymatic flora for drug absorption.9
At prehospitalization and domiciliary care level when IV/IM drug administration is difficult, non conventional routes need to be harnessed. An ideal AED should be safe, effective, easy to administer, rapid acting and cost effective. Rectal diazepam emerged as the primary treatment option for breakthrough seizures in the past. However rectal diazepam has slower onset of action than the intravenously delivered drug and is not as effective at controlling seizures.10 Other disadvantages include low social acceptability and high cost of the commercial preparation.10–12
Intranasal (I/N) midazolam was safe and effective in numerous clinical trials.13–19 On I/N route drug is directly absorbed into the blood and CSF via nasal mucosa. The drug should however be highly concentrated and delivered directly to the mucosa. If the drug is not given in proper position it can go directly into the pharynx rendering it ineffective. The volume limit is about 0.5 ml per nostril hence the practice of using both nostrils. Upper respiratory tract infections may interfere with absorption.20,21 Intramuscular (I/M) midazolam was effective in various trial in controlling acute seizure when I/V access not available but injection is painful to the child so not acceptable socially.23
The oral mucosa allows rapid drug absorption of directly into systemic circulation. However during a convulsion risk for aspiration is serious .The sublingual route is difficult in a tightly clenched jaw during the tonic phase of the seizure.24
The buccal route of BZD (between cheek and gums) studied with midazolam and lorazepam was found effective in controlling acute seizures and does not require the teeth to be parted .Buccal route is more accessible than the rectal route.25–27
An acute breakthrough seizure adds unwanted cost of hospitalisation, burden of investigations & injections besides psychologic stress. Domiciliary Buccal clonazepam possibly reduce hospitalisation, and status episodes.
Subjects were randomized to Buccal clonazepam v/s I/V midazolam comparable for age, sex, seizure type, etiology, abnormal neurological examination, neuroimaging, EEG, duration of acute breakthrough seizure, prior drug intake and other base line characteristics.
Efficacy
The efficacy of IV midazolam and buccal clonazepam in controlling acute breakthrough seizure episode in our study was 92.0% and 86.0% respectively. There was no statistical difference (p=0.175). There is no reported study on buccal clonazepam till date, though the drug has been used by many investigators in treating acute seizure with reported benefit [28-30].This study compared safety, efficacy of buccal clonazepam with a standard of care drug IV midazolam in acute seizures. In our study buccal clonazepam demonstrated efficacy equivalence to IV Midazolam. The easily availability, low cost, easy to use by caregiver make it a practical alternative (shown in Table 2). Larger studied are recommended.
BZDs(Various routes) |
Efficacy |
Cost effectiveness |
Rectal diazepam |
27-100% |
2.5 mg= Rs 30 + cost of drug administration |
Intramuscular midazolam |
93-100% |
10 ml inj= Rs 83+cost of drug administration |
Intranasal midazolam |
85-97% |
Nasal spray =Rs 297 (50 doses) |
Buccal midazolam |
75-87% |
10 ml inj= Rs 83+cost of drug administration |
Sublingual lorazepam |
79.5% |
NA in India |
Buccal clonazepam (present study) |
86% |
0.25 mg = Rs 0.91 (lowest cost) |
Table 2 Comparison of cost and efficacy of various routes of BZD use
NA, not available
Safety
Vital monitoring did not reveal any significant difference in both groups. Total of sixteen (16.0%) adverse reactions were observed after 10 min of drug administration in both groups, four (4.0%) in the IV midazolam group, and twelve (12.0%) in the buccal clonazepam group (p value=0.119). All adverse effects were transient, and no intervention was required.
Recurrence after primary control
Seizure recurrence within 10 min of use is an important aspect not addressed by any study so far with other BZD. In our study recurrence after initial control was Eight (8.0%) in IV Midazolam group and nine (9.0%) in buccal Clonazepam group, the difference was not statistical significance (p=0.800).
Buccal clonazepam demonstrated equivalence to I/V midazolam in controlling acute breakthrough seizure .When extrapolated to domiciliary setting it is likely to be a safe option with capability of decreasing status episodes, hospitalisation and thus reducing economic burden and psychological stress to the family.
This RCT was done at Indraprastha Apollo hospital New Delhi and has been presented at Indian academy of pediatrics (IAP) Delhi branch for Dr Sarla Vaishnavi award in Jan 2012 .Had not been sent to any journal earlier. Authors also acknowledge the help and support. Provided by the Department of emergency Indraprastha Apollo hospital, New Delhi.
ASB, RC, and VK contributed to the study concept and design. SK acquired the data and drafted the manuscript. ASB, RC performed critical reviews of the manuscript. VK acted as supervisor and approved the final manuscript.
Ethical approval
Study was approved by medical ethical committee of Indraprastha Apollo hospital New Delhi.
None.
The authors declare no conflict of interest.
None.

©2016 Kumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.