Journal of
eISSN: 2373-4426


Case Report Volume 9 Issue 4
1Neonatology section, Pediatric Department, Dubai Hospital, UAE
2Pediatric cardiology Department, Dubai Hospital, UAE
Correspondence: Atef Alshafei, MRCPCH, Neonatology section, Pediatric Department, Dubai Hospital, Dubai, UAE
Received: July 09, 2019 | Published: July 23, 2019
Citation: Alshafei A, Ahmed M, Hussein F, et al. A case of neonatal lupus erythematosus with multi-organ involvement and complete heart block. J Pediatr Neonatal Care. 2019;9(4):106-109. DOI: 10.15406/jpnc.2019.09.00388
Neonatal lupus erythematosus (NLE) is a rare autoimmune- mediated spectrum of disorders occurring in 1/20,000 live births and causing fetal tissue damage due to trans placental passage of anti-Sjögren’s-syndrome-related antigens A and B (anti Ro/SSA and anti La/SSB immunoglobulin G). Approximately 60% of the mothers are asymptomatic on diagnosis of NLE, while the remaining may have SLE, Sjögren syndrome, or other autoimmune disorders. Clinical presentation of NLE varies from dermatologic, cardiac, hepatic, splenic, hematologic, or neurogenic abnormalities. All except cardiac manifestations are reversible and benign. We report a case of NLE in a late preterm infant presenting with multi-organ involvement and congenital complete heart block. The mother was completely asymptomatic but had a significantly high anti-Ro/SSA antibody level. Antenatal fetal echocardiography revealed a structurally normal heart with significant bradycardia and complete heart block. After birth, the infant had multi-organ involvement and persistent bradycardia ranging from 45 to 65bpm with respiratory distress secondary to cardiac decompensation. A permanent epicardial pacemaker was implanted at the age of 2weeks with gradual improvement of respiratory and cardiac functions. Upon follow-up, the infant was thriving well and gaining weight with a stable general condition and reasonable pacemaker function at a rate of 100bpm.
Keywords: lupus erythematosus, neonate, heart block, pacemaker, echocardiography
NLE, neonatal lupus erythematosus; CCHB, complete congenital heart block; ENA, anti-extractable nuclear antigen; SLE, anti-smooth muscle, anti-Smith
Neonatal lupus erythematosus (NLE) is an uncommon clinical spectrum of systemic abnormalities described in neonates whose mothers have autoantibodies against Sjögren’s syndrome types A and B autoantigens or both.1 The incidence of NLE is approximately 2% in infants with positive maternal autoantibodies and a recurrence rate of 18-20% in the following pregnancies.2 It is estimated that approximately 25-60% of mothers are asymptomatic when their infants are confirmed to have NLE.3 The transplacental passage of these antibodies may produce significant damage to various developing organs in the foetus which could be irreversible. Cutaneous, cardiac, hematologic, hepatobiliary and nervous systems may be involved. Cutaneous manifestations occur in the form of erythematous patches or plaques typically involve sun-exposed areas, trunk, palms and soles. Although cutaneous lesions are the most common presenting symptoms of NLE, the skin and other organ manifestations are self-limited and commonly reversible by the sixth to eighth month of postnatal life.4 Diffuse myocardial disease occurs in 15-20% of infants with neonatal lupus before birth. However, complete congenital heart block (CCHB) is an uncommon potentially fatal atrioventricular conductive defect with an incidence of 1/20,000 live births. CCHB is affecting the offspring of approximately 2% of women with positive anti Ro/SSA antibodies and the risk rises to 20% if a previous child was affected.5 It is associated with significant mortality and morbidity, being irreversible and occurring early in fetal life between 18 and 24weeks of gestation.6 Cardiac manifestations, including bradycardia due to heart block or other conduction disturbances, are usually noted on antenatal scan or soon after birth. Different trials of medical management for CCHB have been adopted including antenatal intravenous immunoglobulin and corticosteroids, but the results were not favorable.7,8 Epicardial pacemaker implantation in CCHB may be indicated early in symptomatic neonates with decompensated cardiac and/- or respiratory drive with excellent functional outcome.9
A late preterm 36weeks’ gestation female infant was delivered by caesarian section to a P3+0 32-year old woman with an uneventful pregnancy, except for fetal bradycardia with ventricular rate of 57bpm on antenatal scan. Fetal echocardiography before birth confirmed the diagnosis of CCHB and normal cardiac anatomy. The mother was completely asymptomatic; however, screening for possible maternal autoimmune diseases was done based on the antenatal scan. Maternal anti-extractable nuclear antigen (ENA) profiles, including the levels of anti-ribosomal P (Rib-P-protein), anti-smooth muscle, anti-Smith (for SLE), anti-ScL-70 (anti-topoisomerase for scleroderma), and ENA Jo-1(for dermatomyositis) antibodies were less than 0.1 U (negative<0.7 U). Only the anti-Ro/SSA antibody level was 5.78U (positive>1U), while the anti-La/SSB antibody level was 0.137U (negative). The Apgar score was 5 and 9 at 1 and 5min, respectively, and only T-piece resuscitator support was needed for labored breathing. On examination, the birth weight was 2.775 (50th centile); head circumference, 35 cm (50th centile); and length, 50 cm (50th centile). The infant was maintaining saturation on nasal continuous positive airway pressure support with acceptable blood gases; no dysmorphic features and no skin stigmata were observed. Chest examination showed mild subcostal recessions with respiratory rate of 40 -50/min. Heart rate ranged from 60 to 65bpm with grade II systolic murmur over the left upper parasternal border. Femoral pulses were palpable with mean blood pressure of 50-55mmHg in both upper and lower limbs. The liver was palpable about 3 cm below the right costal margin but not tender. The spleen was also enlarged 2-3cm below the left costal margin. Chest X-ray on admission revealed mild cardiomegaly with plethoric well- inflated lung fields (Figure 1). The patient was reviewed by the pediatric cardiology team, and the ECG indicated CCHB with complete PR dissociation (P-P rate 150bpm and R-R rate 57bpm) (Figure 2). Echocardiography showed small PDA, LA/Ao 1.0, functional MR, functional TR, and mild pulmonary hypertension, with dilated right heart.
Initial full blood count showed mild thrombocytopenia of 101,000/mm3. Platelet count decreased gradually to the lowest value of 37,000/mm3 at the age of one week. Then, the number gradually improved back to normal at 314,000/mm3 without intervention at the age of 2weeks. Cranial ultrasonography revealed bilateral linear echogenic streaks at the basal ganglia, suggestive of lenticulo striate vasculopathy (Figure 3). Abdominal ultrasonography showed borderline hepatosplenomegaly but no ascites. Blood chemistry tests including urea, electrolytes, creatinine; liver function tests; and bone profile were all within normal limits. The STORCH profile was negative. At the age of 5 days, atropine injection was administered without improvement of heart rate, and furosemide was commenced in view of development of signs of heart failure clinically and by echo assessment. In the next week, there was progressive increase in respiratory distress with further enlargement of cardiac chambers, and the decision for cardiac pacing was planned. Permanent Medtronic bipolar epicardial pacing leads were implanted on the right ventricle. Pacing leads were connected to St. Jude Microny 2 pacemaker pulse generator placed in a pocket created below the rectus sheath in the left upper abdominal quadrant (Figure 4). Parameters were checked and paced at the 100bpm VVIR mode. The infant was clinically stable after pacing with improvement of respiratory and cardiac function. Repeated ECG showed stable heart rhythm and rate at 100bpm (Figure 5). She was gradually weaned to room air 5days after surgery and discharged home one week later with stable hemodynamic status. Upon follow-up in the outpatient clinic till the age of one year, she was developmentally normal and gaining weight and had normal cardiac functions.
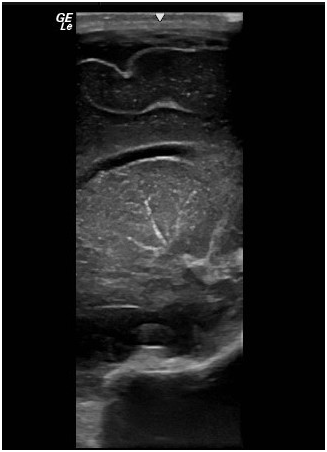
Figure 3 Cranial ultrasonography shows linear echogenic streaks at the basal ganglia suggestive of lenticulo-striate vasculopathy.
NLE represents an autoimmune spectrum of protean manifestations, targeting nearly all body organs. The transplacental passage of anti-Ro/SSA and anti-La/SSB autoantibodies is responsible for fetal tissue damage with variable severity.10 Although cutaneous, hepatobiliary, hematologic, and central nervous systems may be affected, cardiac involvement remains the most serious manifestation of NLE. The mortality rate of CCHB is highest in the fetal and neonatal period with an estimated incidence of 19%. Intrauterine mortality rate is the highest if CCHB is recognized before the 20th week gestation or if associated with hydrops.11 CCHB develops due to inflammation and fibrosis with disruption of the atrioventricular conductive system. However, other fetal factors may be involved like apoptosis of cardiomyocytes and secretion of profibrosing factors and cardiac fibroblast modulation.12 Diffuse myocardial disease such as cardiomyopathy and endocardial fibroelastosis may be seen in NLE without any cardiac conductive disorders.13 Other conductive disorders may include prolonged QT interval, transient sinus bradycardia, and Wolf -Parkinson White syndrome.14 CCHB is usually detected by fetal echocardiography in the second trimester in the view of persistent fetal bradycardia on antenatal scan.15 The majority of these anti- Ro/SSA- and anti- La/SSB- positive mothers give birth to completely healthy infants without any sign of NLE. In first- or second-degree heart block, maternal treatment by steroids, immunoglobulin, and hydroxychloroquine may be beneficial. Once CCHB is established, it is difficult to revert to sinus rhythm despite the use of maternal medications.
A permanent pacemaker placement is usually required in two-thirds of infants with CCHB in the first year of life.16 Early pacing of high- risk neonates could significantly reduce the complications of profound bradycardia and asystole in this subset of infants. The indications of pacing in CCHB include ventricular rate less than 55, prolonged Q-T interval, and heart failure, which is a well-recognized complication in the neonatal period. In our case, poor cardiac contractility and decompensation were evident after the first week, and an epicardial pacemaker was implanted at the age of two weeks with a smooth postoperative course. In most NLE cases, the affected patients usually have isolated manifestation, but this infant had the picture of NLE multi-organ involvement. Besides CCHB, she had hepatosplenomegaly, thrombocytopenia, and lenticulo-striate vasculopathy in the basal ganglia which is occasionally reported in NLE.17
Central nervous system involvement occurs in 1.4% of patients with NLE and may result in long term neurodevelopmental complications in some cases.18 Symptomatic cases may present with focal seizures, hydrocephalus, or spastic paraparesis.19 The anti Ro antibodies may cross the blood brain barrier of affected neonates and attach to Ro antigen expressed in fetal brain causing immune mediated damage to cerebral tissue and choroid plexus.20 Although this patient had excellent recovery with stable heart rate of 100bpm and regression of other organ dysfunctions, yet long-term follow up with close monitoring of cardiac functions by repeated echocardiography and recognition of any evidence of lead failure, lead fracture, or dislodgement is paramount. Children with permanent pacemakers are enjoying different childhood activities without restrictions. The development of biological wireless pacemakers with remote home monitoring would be a reality soon, avoiding the drawbacks of current pacemakers that were designed originally for adult patients.
None.
The authors declared there is no conflict of interest.
None.

©2019 Alshafei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.