Journal of
eISSN: 2373-4426


Research Article Volume 4 Issue 6
1Departments of Pediatric surgery, All India Institute of Medical Sciences, India
2Departments of Pediatric surgery, Vardhaman Mahavir Medical College and Safdarjung Hospital, India
Correspondence: Shilpa Sharma, Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi, India
Received: June 03, 2016 | Published: June 16, 2016
Citation: Mitra A, Sharma S, Yadav DK, Bagga D, Jain V, et al. (2016) Shah-Waardenburg Syndrome: A Spectrum of Aganglionosis. J Pediatr Neonatal Care 4(6): 00158. DOI: 10.15406/jpnc.2016.04.00158
Aim: To report our experience with four neonates with Shah-Waardenburg syndrome (SWS) and highlight the spectrum of intestinal aganglionosis in this disorder.
Method: Four consecutive cases of SWS that were managed over last five years at two pediatric surgical centres are described. The syndrome was identified by virtue of its various components.
Results: All four children (3 boys/1 girl) presented in the neonatal period with features of intestinal obstruction. All had the characteristic white forelock and skin depigmentation with blue/grey irides. Two children were diverted (right transverse colostomy and ileostomy respectively) which never functioned satisfactorily. They died due to septicemia before further procedures could be planned. One child underwent three laparotomies with progressively proximal stoma construction. Eventual jejunal biopsy was also aganglionic and guardians chose to terminate therapy. The fourth child underwent an ileostomy in the neonatal period for total colonic aganglionosis and later on, an ileal pull-through. He is thriving on follow-up of 4 years.
Conclusions: The striking association of phenotypic features makes SWS a rare but highly recognizable syndrome. Evaluation for Hirschsprungs’ disease must be done in such children while keeping a high suspicion for the extended varieties.
The Waardenburg syndrome is a neurocristopathy which stems from the arrested migration of neural crest cells. It is a rare disorder with an overall incidence of 1 in 40,000-50,000 live births. It has four types. Among the types, types I and II are frequently seen. However, types III and IV are extremely rare. Type 4 of the spectrum, the Shah-Waardenburg syndrome (SWS) is characterized by the addition of intestinal aganglionosis1 to a constellation of hypopigmentation, heterochromia irides, depigmented ocular fundus, telecanthus, and sensorineural hearing loss.2 It is thus Waadenburg syndrome with Hirschsprungs’ Disease (HD). Type 4 is rare with only 48 cases reported up to 2002. What makes the presentation so variable is the spectrum of aganglionosis seen in those afflicted. Some authors have drawn parallels between the length of the affected segment and the age at presentation.3 Although the identification of these striking phenotypic features has always been a red-flag for the possibility of associated total colonic aganglionosis (TCA), this series of four children clearly demonstrates the variability in length of involved bowel.
Case 1
A term born male infant with a birth weight of 2430 g presented on day 3 of life with abdominal distension and bilious vomiting. He had not passed meconium since birth. A family history was not contributory. Contrary to the expected dark iris pigmentation of the local population and his parents, the baby had bilateral light grey irides along with a white forelock and generalized patchy hypopigmention. A non-ionic contrast enema was not suggestive of a classical rectosigmoid transition zone. An exploratory laparotomy was subsequently planned. The operative findings suggested total colonic aganglionosis and an ileostomy was created after collecting multiple seromuscular biopsies. The ileostomy did not function well in the post-operative period. He developed abdominal distension and succumbed to septicemic shock by the fourth post-operative day. The colonic, ileal biopsies and appendix were aganglionic.
Case 2
A term-born 2600 g male infant presented on day 4 of life with abdominal distension and vomiting. He had passed a small amount of meconium only after rectal stimulation on day 2 of life. A family history was not contributory. The irides were heterochromic – left was blue and right was grey- along with a white forelock. Distinct hypopigmented patches were noted on the elbows and thighs. A non-ionic contrast enema was suggestive of a poorly distensible rectum and a doubtful transition zone. A right transverse colostomy was made with a suspicion of Hirschsprung disease. The stoma functioned poorly in the first two post-operative days that was related to a probable transition zone stoma. Re-exploration with a proximal stoma was planned. However, the baby developed septicaemia and thrombocytopenia with a progressive deterioration and eventual died. The retrospective review of biopsies revealed aganglionosis with hypertrophic nerve bundles in the supposedly normal looking bowel at laparotomy.
Case 3
A 2400 g term born female child presented on day 2 of life with progressive abdominal distension and bilious vomiting. An exploratory laparotomy conducted at another hospital had revealed dilated small bowel with a collapsed colon. A Ladd’s procedure had been done for malrotation and multiple seromuscular biopsies had been taken. The child was referred to us in view of recurrent abdominal distension with continued high NG output. A white forelock with hypopigmented eyelashes and grey irides were evident at presentation (Figure 1). Interestingly, the cutaneous hypopigmentation was patchy at presentation and later became progressively confluent over time. The father had been operated successfully for anorectal malformation in the past. She underwent an ileostomy in view of suspected total colonic aganglionosis (TCA). The the stoma did not function in the post-operative period. The biopsies were aganglionic. Another exploration with frozen section was done and a more proximal jejunostomy was fashioned accordingly. However, the stoma did not function well again and the histopathology of the jejunal segment was also aganglionic. Total gut aganglionosis was suspected and the guardians chose to terminate therapy at this point.
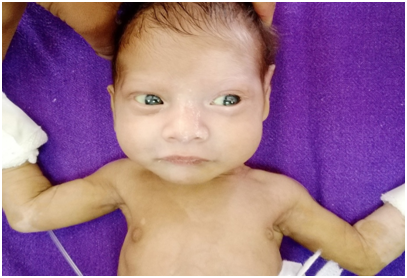
Figure 1 Hypopigmented (grey) iris, white forelock, poliosis, broadened nasal root and piebaldism is evident.
Case 4
A 3100 g male infant born at term presented on day 5 of life with abdominal distension since day 1 of life and recurrent bilious vomiting. There was no history of spontaneous passage of meconium. The characteristic white forelock and hypopigmented irides were present. Faintly hypopigmented patches over the body showed a progressive loss of pigment over time as in case 3 (Figure 2). A contrast enema revealed a uniformly small calibre colon with poor rectal distensibility. An exploratory laparotomy suggested total colonic aganglionosis and an ileostomy was then fashioned after taking the requisite seromuscular biopsies. The child thrived in the post-operative period and eventually underwent ileal pull-through for total colonic aganglionosis. He was thriving well at a follow-up of 4 years.
Shah-Waardenburg syndrome remains a curiosity for neonatologists and pediatric surgeons due to the ability to make a diagnosis based on clinical features and associated poor prognosis due to the involvement of a larger segment of bowel with aganglionosis.
The diagnosis of a baby with this auditory-pigmentary syndrome requires the fulfilment of at least two major or one major and one minor criterion according to the Waardenburg consortium.4 Three putative genes have been identified including EDNRB, EDN3 and SOX10.5 Although the prevalence is ill-defined, about 50 cases had been reported till 2015.6
The aganglionic segment has been reported to be long in these children and amounts to a poor prognosis. Our series included two children with proven TCA, one child with total gut aganglionosis and another with a possible long segment HD. The latter died of sepsis before he could be taken up for the revision of a poorly functioning/transition zone right transverse colostomy. A higher than normal incidence of gastroenteritis and enterocolitis has been noted in these children7 and was also evident in our group of patients (two of whom developed enterocolitis and fulminant sepsis).
As regards the phenotypic manifestations, a review of six cases by Jan et al.8 concluded that HD should be ruled out in any baby with white forelock and cutaneous depigmentation. Considering the rapid course of enterocolitis and the hurdles of administering medical care in a resource challenged nation, widespread sensitization of primary care physicians and paediatricians is essential to ensure early referral. These striking phenotypic features (Table 1) allow the early identification of affected neonates.
Feature |
Cases of Shah-Waardenburg syndrome described |
|||
1 |
2 |
3 |
4 |
|
White forelock |
+ |
+ |
+ |
+ |
Blue Iris |
+ |
+ |
+ |
+ |
Heterochromia irides |
- |
+ |
- |
- |
Skin Hypopigmentation |
+ |
+ |
+ |
+ |
Deafness |
likely |
likely |
likely |
Present |
Aganglionosis |
+ |
+ |
+ |
+ |
Segment involved with aganglionosis |
TCA* |
Long segment |
TCA/ ?Total gut |
TCA |
Table 1 Features of Shah-Waardenburg syndrome in presented series
*TCA- Total Colonic Aganglionosis
The hypopigmented irides and white forelock were universally present. A white forelock may take time to become obvious in babies.8 Two of our children demonstrated gradual loss of pigment over time and the leukoderma evolved during the stay in the neonatal ICU (Figure 3). Heterochromia was seen in one patient and is not considered an essential criterion. Sensorineuronal hearing loss requires the brainstem evoked response audiometry (BERA) test for confirmation. Although it was not feasible to do the BERA in the three newborns, the absence of reflexive activity to loud sounds and attentive behaviour to softer auditory stimuli did portend the presence of profound hearing loss in our babies.
Contrast enema studies are usually not characteristic in the rectosigmoid variety and microcolon may be seen in those with TCA. Exploratory laparotomy becomes mandatory in order to take appropriate tissue specimens for histopathologic diagnosis. In one of our cases, malrotation was an association and was tackled with Ladd’s procedure (Case 3). This association is quite rare with extensive aganglionosis9 and has never been described with SWS.
Appropriate management should address the initial bowel decompression while being aware of the implications of diversion at the level of ileum or higher. The long aganglionic segments necessitate the creation of such high output stomas. Fluid/electrolyte imbalances, complications of total parenteral nutrition and sepsis must be countered effectively. Survivors are likely to have short bowel and the surgeon needs to be aware of the myriad definitive procedures described in order to customize the therapy to the patient. Modified Duhamel, Kimura-Stringel and ileoanal pull-through with Soave techniques have been described with varying degrees of success.9
The striking association of phenotypic features makes SWS a rare but highly recognizable syndrome. Evaluation for Hirschsprung disease must be done in such children while keeping a high suspicion for the extended varieties. A higher morbidity and mortality is documented in these babies thus, early intervention is essential to prevent the inevitable fulminant enterocolitis and sepsis. A guarded prognosis needs to be explained to the parents.
None.
The authors declare there is no conflict of interests.
None.

©2016 Mitra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Down Syndrome Day is observed on 21 March 2026 to raise awareness about Down syndrome and to
promote inclusion, early intervention, and quality healthcare for children with genetic conditions. This day highlights the importance
of pediatric monitoring, developmental support, and continued research to improve health outcomes and overall well-being.
Researchers, pediatricians, and healthcare professionals are invited to submit their original research articles, reviews, and clinical
studies related to Down syndrome, neonatal screening, developmental pediatrics, and child health. Manuscripts submitted on the occasion
of World Down Syndrome Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
World Down Syndrome Day is observed on 21 March 2026 to raise awareness about Down syndrome and to
promote inclusion, early intervention, and quality healthcare for children with genetic conditions. This day highlights the importance
of pediatric monitoring, developmental support, and continued research to improve health outcomes and overall well-being.
Researchers, pediatricians, and healthcare professionals are invited to submit their original research articles, reviews, and clinical
studies related to Down syndrome, neonatal screening, developmental pediatrics, and child health. Manuscripts submitted on the occasion
of World Down Syndrome Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).