Journal of
eISSN: 2373-6445


Brief Report Volume 14 Issue 2
Colleges of Medicine and Graduate Studies, University of Science Arts and Technology, Montserrat, BWI
Correspondence: Orien L Tulp, Professor, Colleges of Medicine and Graduate Studies, University of Science Arts and Technology, Montserrat, British West Indies
Received: April 01, 2023 | Published: April 14, 2023
Citation: Tulp OL. Effect of the obese phenotype on brain composition in congenic lean and obese LA/Ntul//-cp rats. J Psychol Clin Psychiatry. 2023;14(2):38-43. DOI: 10.15406/jpcpy.2023.14.00726
To determine if the obese phenotype impacted on brain development and composition, groups of congenic male lean and obese littermates of the LA/Ntul//-cp rat strain were fed on a standardized nutritionally adequate diet in littermate pairs from weaning until adulthood. The obese phenotype of his strain develops early onset chronic hyperinsulinemia associated with hypertrophic-hyperplastic obesity during early postweaning growth. At 10.5 months of age, animals were sacrificed by cervical dislocation, and brain tissues excised in its entirety, weighed to the nearest mg, and measures of protein, DNA and lipid determined. Body weights (BW) of obese were significantly greater than lean. Brain mass (BrM) of lean > obese, and BrM:BW of lean was greater obese. Brain protein content (BPC) and Brain DNA (BDNA) of lean was greater than obese. Brain lipid as a percent was similar in both phenotypes and net brain lipid content was proportional to brain mass. Total body fat mass of obese was significantly greater than occurred in lean littermates. Inflammatory cytokines residing in adipose tissue have been reported to contribute to DNA damage in neuronal and other tissues, impede cell replication, and accelerate cell senescence. These results indicate that brain growth and cellular development is impaired in the hyperinsulinemia-prone obese phenotype of this strain, and are likely associated with development of a chronic inflammatory syndrome and cytokine expression common to excessive fat accretion and obesity.
Keywords: Obesity, brain development, hyperinsulinemia, DNA, rat
The prevalence of hypertrophic-hyperplastic obesity is rapidly approaching epidemic proportions in Westernized society.1 In addition, chronic obesity and nutritional factors have been linked to premature dementia including Alzheimer’s disease in addition to impaired neurodevelopment and DNA damage in multiple tissues when it occurs in earlier, formative life stages of growth and development and in aging.2,3 The syndrome of insulin resistance, glucose intolerance, and chronic inflammation typically accompanies obesity in man and animals, often resulting in increased comorbidities and decreased life span when left untreated.4,5 The mechanisms contributing to chronic insulin resistance have been attributed to multiple factors, including chronic hyperphagia and other dietary macronutrient imbalances, disordered glucocorticoid metabolism and actions, impaired cellular translocation of insulin-dependent GLUT4 transporters, and immune dysfunctions.6–8 Macrophage infiltration of adipose tissue, often resulting in disordered immune functions is one of the earmarks of obesity-mediated immune dysfunction.9,10 Among the immunologic dysfunctions, adipose tissue tends to attract macrophages, which lead to secretion of inflammatory cytokines including IL6 and others, that may now circulate systemically and impinge on numerous central and peripheral tissues, where they may initiate pathophysiologic sequela including vascular lesions and likely microglial contributions to premature apoptosis of neuronal cells.9–12 In humans, the neuronal changes in obesity plus Alzheimer’s syndrome include shrinkage of brain volume and cognitive deficits that accompany the increased adiposity.13 Thus, the purpose of the current study was to determine key parameters of brain composition in older, congenic lean and hyper insulinemic obese male rats, and to correlate the changes in brain composition with the magnitude of adiposity.
Optimal nutrition has been shown to influence pre- and postnatal growth and to be positively associated with multiple developmental parameters that may follow later in life in man and animals.14 In contrast, early malnutrition and undernutrition are often followed by reduced stature and other developmental parameters later in life, proportional to the magnitude of the nutritional depravation. Brain development can also be impacted by early nutritional factors, and in the most dire examples with reduced brain development and decreased brain DNA content.14 In epigenetic rodent obesity, however, pre- and early postnatal nutrition are presumed to be comparable to similarly reared lean littermates in that they typically share the same lactation and equal access to solid food by weaning and thereafter when fed and housed under standard laboratory conditions.15,16 Thus, deficits in a congenic animal model are unlikely to be associated with inadequacies in nutritional deficits, but may be associated with the hyperphagia and resulting metabolic sequela that accompany the epigenetic expression and subsequent development of adiposity and obesity.14
The epigenetic expression of obesity in the LA/Ntul//-cp (corpulent) rat strain occurs as the result of an autosomal recessive trait, and results in 25% of the offspring of heterozygous breeding pairs demonstrating early stigmata of the progressive development of obesity by 5 to 6 weeks of age.15–19 The obese phenotype demonstrated hyperphagia, hyperinsulinemia, hyperamylinemia, hyperlipidemia including hypertriglyceridemia, and impaired glycemic responses to a glucose tolerance within a few weeks of postweaning life.15,20,21 In this strain, however, the obese littermates develop impaired glycemic responses typical of peripheral insulin resistance, but to date have remained non-diabetic throughout their lifespan.20 The animals have remained specific pathogen free (SPF) in an isolated colony throughout many generations in our laboratory. Thus, the primary aspect of metabolism that remains is the chronic, lifelong hyperinsulinemia and hyperamylinemia and their pathophysiologic sequela.15,16,21
Animals were housed in large plexiglass cages lined with one inch of pine shavings, maintained at 22°C and 50% RH under standard housing conditions in littermate pairs. The only known difference between the lean and obese phenotypes was the -cp trait for obesity, originally obtained from the Koletsky rat18 and backcrossed into the longevity prone NIH LA/N background strain for 12 cycles by Hansen to establish the congenic designation at the NIH.17,19 Rats were maintained on Purina chow (#5012) and free access to house water from weaning. At 6 weeks of age rats were placed on a nutritionally adequate diet containing 54% carbohydrate as cooked cornstarch, 20 % protein, and 16% mixed fat plus essential vitamins, minerals and fiber as described by Michaelis et al.20 At 10.5 months of age animals were sacrificed by cervical dislocation with a small animal guillotine after a brief 4 hour fast and cervical blood was obtained for later analysis. The brain tissue was dissected and weighed to the nearest mg. and 50-75 mg aliquots of representative samples taken for proximate analysis. The residual carcasses including the remaining brain tissues were frozen, homogenized in a Waring blender, lyophilized and subjected to gravimetric analysis for determination of protein and lipid content.22,23 Measures of protein content of brain and residual carcass were obtained with the methods of Dole and Lowry22,23 and DNA analysis in brain tissue by the method of Burton.24 Data were analyzed by standard statistical procedures.25 The study was approved by the Institutional Animal Care and Use Committee.
The results of final body weights obtained on the morning of the day of dissection are depicted in Figure 1, and indicate that by 10.5 months of age, the obese phenotype weighed more than twice the weights of their lean littermates, despite having been reared identically with respect to both diet and environment, with ad libitum access to a nutritionally sound diet. The brain wet weights and the brain weight to body weight ratios are depicted in Figure 2 and indicate that the brain weights of the obese rats were significant less than their lean littermates at 10.5 months of age (p<0.05). In addition, the ratio of brain weight to final body weight of obese animals was also significantly less than their similarly reared lean littermates (p=<0.01). Brain total protein and DNA content are depicted in Figure 3 and indicate that measures of both net protein and DNA content per brain were decreased in the obese phenotype. (p=<0.05 for both measures). Brain lipid content is depicted in Figure 4 and indicate that although the amount of lipid per brain was less in the obese phenotype, the percentage of lipid content in the brains were similar in both phenotypes, suggestive of a proportional decrease in overall brain composition rather than a decrease in any specific chemical component. Thus, although the brain lipid mass was lower in the obese than the lean phenotype, the results are consistent with and correspond to the small brain mass observed in those animals. When the net brain mass is compared to final body weight, the proportion of brain tissue to total body weigh was significantly less in the obese than the phenotype, reflecting the substantially greater adiposity and body mass of the obese phenotype.
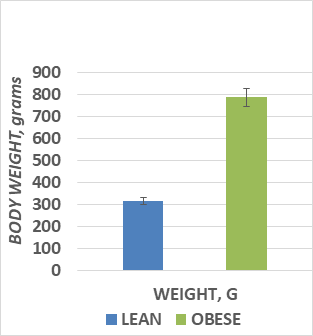
Figure 1 Live body weights of rats in grams at 10.5 months of age.
Data are mean ± 1 SEM, n=8 rats/group. P=<0.01 by Students t test
.
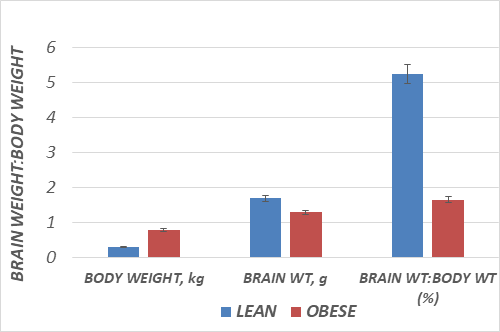
Figure 2 Brain weights of rats, in grams per brain. Data are mean ± 1 SEM, n=8 rats/group. P=<0.05 by Students t test .
The results of this study indicate that at 10 months of age, total brain weight and absolute lipid, protein and DNA content were decreased in the obese phenotype, despite having been reared since birth with biological littermates via the same nutritional and environmental conditions. The study did not monitor daily caloric intake, but numerous previous reports have demonstrated hyperphagia throughout much if not all of the lifespan in the obese phenotype of this strain, and where the typical duration of the lifespan of the obese phenotype is about one third shorter than its similarly reared and housed lean littermates.15,26 Previously, brain size and cellularity have been shown to be decreased under conditions of severe malnutrition, and in humans that develop Alzheimer’s disease, but only limited reports tend to identify obesity as an isolated contributor to premature brain shrinkage. The decreases in brain DNA content in the present study indicate decreased neuronal cellularity, and presumably decreased cognitive functions as well and may accompany the decrease in cell number. Although the animals were not subjected to cognitive evaluation, and the decreased brain size and cellularity could not be directly correlated with cognitive decline in the present study. They do however permit an assumption of decreased cognitive potential, and one where the presence of inflammatory cytokines may be considered as a contributing factor in the neurologic decline. In addition, since brain measurements were only undertaken at age 10.5 months, the chronologic timeline of the progression of neurologic decline could not be established. Unlike dietary induced forms of obesity, where return to the normal diet is often associated with weight loss, the obese phenotype in this epigenetic strain has to date proven to be remarkably refractory to significant weight loss, or a return to the habitus of their lean littermates following dietary intervention.27 To date, only excess daily T3 but not T4 administration has demonstrated significant weight loss in the obese phenotype, with adrenalectomy resulting in partial but incomplete recovery.27
The physiologic or metabolic basis for the differences in brain weight and composition are unclear, but are highy suggestive of chronic hyperinsulinemia as contributing factors. Elevations in plasma insulin have been reported to occur through much if not the entire lifespan of the obese phenotype of this strain, and which contribute to the impaired thermogenesis in response to factors of diet and environment, including insulin inhibition of UCP1 actions in brown adipose tissue (BAT).28–30 The BAT is a primary tissue in expressing nutritionally and environmental increases in thermogenesis, and when impaired due to metabolic aspects of insulin resistance in BAT and other peripheral tissues, results in increased rates of lipogenesis and body fat accretion in both visceral and subcutaneous adipose tissue depots.29,30
In lean animals, white adipose tissue (WAT) is enriched with Type 2 immune cells, which interact with each other to generate Type 2 cytokines including IL-4, IL-5, and IL-13 to maintain a healthy type 2 protective physiologic environment. In contrast, with the development of obesity and in the presence of hyperinsulinemia,9,10 and may promote development of a Type 1 inflammatory response, including the generation of inflammatory cytokines IL-6 and others which may produce damaging effects on neuronal DNA and neuronal survival. The type 1 inflammatory response includes metabolically activated macrophages, T-cells, B cells and others. The activated macrophages and other immune cells can collectively induce a Type 1 inflammatory environment. The inflammatory cytokines can now migrate to virtually all somatic and neuronal tissues, where they contribute to cellular senescence. Dietary factors including excess fatty acids common to hypertriglyceridemia and obesity in concert with the hypoxia that occurs in WAT have been shown to contribute to a chronic low-grade inflammation that can induce the pathophysiologic sequela including premature apoptosis among neuronal tissues, similar to that which occurs in Alzheimer’s disease.9,13
The role of glucocorticoids in the development of obesity may follow several lines of evidence. Glucocorticoids can induce both anti-inflammatory and inflammatory immune responses particularly from macrophages, which can generate both Type 1 (secretes IL-6, inflammatory) and promote type 2(anti-inflammatory) responses.2,3,31,32 The physiologic response of glucocorticoids tend to be counterregulatory to those of insulin, creating a somewhat vicious cycle that may only become further aggravated in the presence of a chronic hyperinsulinemic state and which help to discern the impaired modulation of immune responses.2,7,28 Among other processes, dysregulation of glucocorticoid actions impede the formation and cellular translocation of GLUT4 glucose transporters from the endoplasmic reticulum of somatic cells, resulting in impaired cellular glucose uptake, thereby spiking further increases in insulin release to insulin dependent tissues, while promoting lipogenesis in liver and other receptive tissues.7,8 Indeed a hallmark characteristic genetically-obese rats if the progressive development of a fatty liver with advancing age, and which were visually apparent in all of the obese rats of the present study that were dissected.15–17 Although glucocorticoids induce cell death and reduce cell survival in immune cells such as T and B cells, macrophages in most tissues are relatively resistant to glucocorticoid-induced apoptosis. Overall, while glucocorticoids act to suppress systemic inflammation and are frequently prescribed to treat chronic inflammatory condition involving lymphocytes, they are less effective in macrophage-mediated diseases, such as chronic obstructive pulmonary disease and the chronic inflammation and hypoxia of obesity where an elevated release of inflammatory cytokines may occur.32–34 Regardless of the cellular processes implicated, the brain size, absolute composition, and cellularity based on DNA content is reduced in the aging adult obese non-diabetic phenotype of the LA/Ntul//-cp rat.
The results of the present study indicate that brain mass and cellular content at 10 months of age is decreased in the hyperinsulinemia-prone obese phenotype of this strain, and are likely associated with a chronic inflammatory syndrome and cytokine expression that are common to obesity. The results could not determine the chronology of development the decrease or confirm an etiologic or developmental origin in brain size or composition as the data reflect only a single time point taken during late adulthood, in animals that typically often only survive for 12 to 15 months due to pathophysiologic complications of their obesity. In contrast the lean littermates have been observed to survive for 2 years or longer under similar environmental conditions, with females exhibiting a lesser magnitude of hyperinsulinemia and glucose intolerance, and surviving longer than males. The decreased brain size was characterized by proportionate decreases in total lipid, brain protein and brain DNA content, in association with marked body fat accretion, obesity and a decreased brain to body weight ratio in the epigenetic obese (-cp/-cp) phenotype of this congenic rat strain. The decreases in brain size and cellularity are consistent with the brain shrinkage that occurs in Alzheimer’s Disease and other states of dementia where inflammatory cytokines may prevail and that occur in aging humans, but the biological mechanisms or chronology through which the changes in brain composition occurred in the present study could not be determined from the single data point obtained. Inflammatory cytokines of the macrophage-generated Type 1 category include IL-6 and others, and have been reported to generate free radical damage to DNA and contribute to neuronal senescence, and thus remain an interesting causal speculation from the data obtained. Regardless of the biological mechanism involved, the brain mass and apparent cellularity was significantly decreased in the obese phenotype of this rodent strain.
The author wishes to thank the late Dr. Otho E. Michaelis IV of the Carbohydrate Nutrition Research Laboratory, Beltsville MD Human Nutrition Institute, Beltsville MD USA for the generous contribution of the experimental diet formulas used in this study, and to Mr. Peisong Huang for his very capable assistance in animal husbandry and data collection.
None.

©2023 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Eating Disorder Week is observed from 23 February 2026 to 01 March 2026 to increase awareness of eating disorders and their psychological impact, and to promote early intervention and recovery. This initiative highlights the role of psychology and clinical psychiatry in understanding and treating eating disorders.
Researchers are encouraged to submit relevant research articles, reviews, and clinical findings. Submissions received during this week will receive a 30–40% publication discount in the Journal of Psychology & Clinical Psychiatry (JPCPY).
.
World Eating Disorder Week is observed from 23 February 2026 to 01 March 2026 to increase awareness of eating disorders and their psychological impact, and to promote early intervention and recovery. This initiative highlights the role of psychology and clinical psychiatry in understanding and treating eating disorders.
Researchers are encouraged to submit relevant research articles, reviews, and clinical findings. Submissions received during this week will receive a 30–40% publication discount in the Journal of Psychology & Clinical Psychiatry (JPCPY).
.