Journal of
eISSN: 2373-6410


Case Report Volume 2 Issue 3
1Neurosurgery Department, Hautepierre University Hospital, France
2ICU Department, Hautepierre University Hospital, France
3Neurosurgery Department, Gilbert, USA
4Neurology Department, Hautepierre University Hospital, France
Correspondence: Abid Houssem, Neurosurgery Department, Hautepierre University Hospital, Strasbourg, France
Received: April 29, 2014 | Published: May 8, 2015
Citation: Houssem A, Jean-Etienne H, Marco M, et al. Unusual and dramatic presentation of “adem”: what could be done in neurosurgical practice? J Neurol Stroke. 2015;2(3):45-49. DOI: 10.15406/jnsk.2015.02.00053
The authors describe the case of a young patient who presented with new onset of acute disseminated encephalomyelitis responsible for progressive unilateral brain edema causing trans-tentorial herniation. The patient underwent urgent standard right hemispheric decompressive craniectomy; unfortunately this was not successful and the patient had rapid neurological demise.
Keywords: Acute disseminated encephalomyelitis, Cerebral edema, Hemicraniectomy
ADEM, Acute disseminated encephalomyelitis; ICU, Intensive Care Unit; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; ICP, Intracranial Pressure; CNS, Central Nervous System
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating immune-mediated disorder more common in the pediatric population. It is often associated with either viral or bacterial infection or it may be seen as rare post vaccination complication. It is characterized by an inflammatory reaction and demyelination of the central nervous system (CNS), with pathological changes occurring typically around small veins.1
The demyelination may occur anywhere in the brain, accounting for the wide spectrum of its clinical manifestations and disease severity. The pathophysiology involves transient autoimmune response directed to myelin or other self-antigens, or by nonspecific activation of auto-reactive T cell clones.2,3
Although some patients present with minor focal CNS abnormalities, others have a more rapid and extreme presentation. Acute clinical presentation is characterized by a rapid onset of encephalopathy, multifocal neurological deficits, convulsions, and impaired consciousness with fever. There are no biological markers of the disease.
Generally considered to be an autoimmune response against CNS myelin-associated antigens.4 the mainstay of medical treatment is focused on immunosuppression and supportive therapy. Although high-dose corticosteroids are most commonly used in the treatment of ADEM, other therapies have been used alone or in association including cyclophosphamide, intravenous immunoglobulin and plasmapheresis.3-5 The indications for surgical treatment remain still controversial.
Rarely, ADEM may cause severe brain swelling responsible for intracranial hypertension.6 in case of brain swelling with raised intracranial pressure (ICP), refractory to conventional medical management, surgical decompression has been advocated as a “last resort form of treatment”.6,7 The reversible nature of most of the deficits caused by ADEM justifies an aggressive management to avoid irreversible injury and death due to extensive brain swelling and its related consequences.
Brain and spinal MRI are the imaging modality of choice to point out white matter changes in ADEM. In this paper the authors describe how dramatic evolution of ADEM may be; also, relevant issues such as clinical features and possible management in neurosurgical practice along with pertinent literature will be addressed.
We report the case of a 32-year-old woman, with no relevant medical history, who presented with sudden onset of left hemiparesis following pneumonia. Following admission to the Neurological ward an urgent MRI showed an isolated right fronto-parietal lesion, hypointense on T1, hyperintense on T2 with mild mass effect on the surrounding brain structures as well as multiple micro- hemorrhagic lesions with peripheral contrast enhancement and a smaller second left frontal localization (Figure 1).
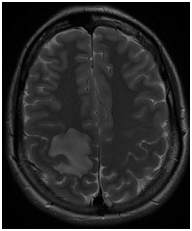
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion. In the first instance the hypothesis of a pseudo-tumoral multiple sclerosis was considered and steroids were started. Follow up CT completed 24 hours later showed however increased mass effect on the right occipital horn although the patient seemed to do relatively well from a clinical standpoint. Corticoids were reduced (Figure 2).
Follow up CT completed 24 hours later showed however increased mass effect on the right occipital horn although the patient seemed to do relatively well from a clinical standpoint. Corticoids were reduced (Figure 2).
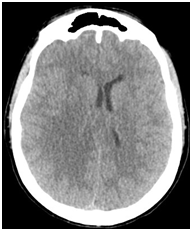
Figure 2 Axial non-enhanced CT scan (24 hours check) showing a right fronto-parietal edema with mass effect on lateral ventricle and midline shift.
Forty-eight hours later however the patient develop a sudden neurological deterioration and she was noted to be more somnolent; follow up brain MRI was repeated and showed significant enlargement of the previous lesion with relevant edema and midline shift. Higher steroids doses were administered and, once again good functional and clinical amelioration was noted (Figure 3 & 4).
At day 5 steroids were weaned off but within 24 hours, the patient started to complain of severe headache; in addition she became progressively drowsy and right mydriasis was noted as well; at this stage an urgent CT-scan showed massive right fronto-temporo-parietal edema with significant midline shift and right trans-tentorial herniation (Figure 5 & 6).

Figure 5 Axial non-enhanced CT scan showing massive brain edema with midline shift (120 hour from admission).
Her clinical condition rapidly deteriorated within 30 minutes and she developed bilaterally dilated unreactive pupils; she was then taken immediately on the operating room and underwent urgent decompressive right hemicraniectomy; the procedure allowed massive brain herniation rendering scalp closure very difficult. This actually required right frontal and temporal lobectomy as well as temporalis muscle amputation. Despite the procedure, the patient expired 36 hours after the operation. During surgery random biopsies were performed showing features in favor of ADEM, which was also been confirmed by autopsy.
ADEM is an inflammatory immune-mediated condition more commonly observed in children after a common viral or bacterial infection or vaccination. ADEM is quite rare in adults and its differential diagnosis includes malignancy, infection as well as other demyelinating disorder.1,8 In 2007, the International Pediatric Multiple Sclerosis Study Group defined ADEM as "the first clinical event with a poly-symptomatic encephalopathy, with acute or subacute onset, showing focal or multifocal hyperintense lesions predominantly affecting the CNS white matter." This definition shows how difficult it may be to differentiate ADEM from a multiple sclerosis at an early stage.8
Pathophysiology remains still unclear and it is probably due to a transient autoimmune response against myelin or other self-antigens.2,3 Brain biopsy is rarely done and performed in difficult and selected cases.9,10 Histological features of ADEM include perivenous demyelination and lymphocytic, plasma cells and monocytes vessel wall infiltration.11
Only few case of clinically aggressive forms of ADEM have been reported, thus knowledge about the best management of this condition is lacking.12 Although immunosuppressive therapy is the mainstay of treatment, the slow improvement leaves room for abrupt and malignant progression of cerebral edema within the first week of treatment, as it happened in our case. Our patient developed progressive and severe brain swelling with trans-tentorial herniation which did not respond to steroids and conservative treatment. In our interdisciplinary meeting the case was largely discussed and debated with neurologist.
Consensus was that maybe steroids were weaned off too early especially in consideration of the discrepancy between clinical and radiological findings; while the patient improved and relapsed twice from a clinical standpoint, CT and MRIs showed constant progression of the lesions indicating an extremely aggressive pathological process probably refractory to therapy.
From a diagnostic point of view CT scan represent a useful investigation showing usually white matter enhancing hypodense lesions which increase progressively in size. MRI remains however the gold standard imaging in suspected ADEM, although findings can vary a lot: Classically FLAIR sequences show multiple white matter, asymmetric hyperintense areas.8,13 more frequently involving thalami, basal ganglia, and periventricular white matter.14-16 Enhancement of T1-weighted images is not constant.8
Management of ADEM is currently well established.17 In the acute stage, it includes high-dose of intravenous steroids, intravenous immunoglobulin, and plasmapheresys .2 Corticosteroid as methylprednisolone is the first-line (10-30 mg/kg/day, up to a maximum of 1 g/day).2 If aggressively treated, ADEM has a good prognosis and more than 50% of the patients achieve total functional recovery.18 while the remaining 50% may suffer mild neurological deficits .2,18
In the majority of cases, increased ICP can be managed by conservative treatment, with a combination of the usual measures such as 30° head elevation, mannitol therapy, hypertonic fluid infusion, brief and relative hyperventilation, and mild hypothermia. When ICP remains elevated despite conservative treatment, the remaining options may also include barbiturate coma and/or decompressive craniectomy.
Patient ICU admission with airway protection is always necessary. A crucial point is also an accurate management of the electrolytes and metabolism maintaining correct fluid balance. If seizures develop, specific anti-epileptic treatment is required. In case of failure of all available conservative treatment, surgical decompression of a swollen brain may represent the last resource. The potential benefits of this procedure in decreasing ICP and relieving/impending herniation with brainstem compression have been increasingly reported for stroke and trauma. Although timing and patient selection for decompression remain controversial, anecdotal case series and some prospective studies suggest that adults with brain swelling from large hemispheric infarctions may benefit from decompressive craniectomy.19-25
Mortality in ADEM varies from 1 to 12%.2,18,26,27 and refractory raised ICP remains the primary cause of death. Decompressive craniectomy in ADEM has been very rarely reported in the international literature and up-to-date only 5 cases are described in current literature to our knowledge.7,26,28-30 Interestingly it appears that all patients had a favorable outcome with a GOS of 4 or 5 (Table 1).
Case no |
Authors |
Sex |
Age Year/ Month |
Symptoms |
Duration |
History of Recent Infection |
Outcome |
1 |
Von Stucckrad, 2003 |
♀ |
34 y |
Fever+headache |
Since 6 days |
No |
Complete clinical recovery |
2 |
Daniel Refai, 2005 |
♀ |
51 y |
Subacute onset of fatigue+spatial desorientation+headache+left hemiparesis |
Since 2 days |
Yes |
Discharged home 6 weeks after intervention |
3 |
Nilsson, 2009 |
♀ |
50 y |
Partial epileptic seizures+dysdiadochokinesis of her right arm+dysphasia+right sided facial palsy |
Since 14 days |
Yes |
After 6 weeks: very mild dysphasia+slight paresis in the right arm |
4 |
Ahmed, 2010 |
♀ |
38 y |
Headache, vomiting, left sided weakness |
Since 1 week |
Yes |
Discharged home on day 11. |
5 |
Granget, 2012 |
♀ |
18 m |
Left hemibody seizures fever |
Few hours |
Yes |
Two years later, discrete right hemiparesis |
6 |
Present case |
♀ |
32 y |
Left hemiparesis |
Few hours |
Yes |
Dead |
Table 1 Reported cases in the English literature
Pathological feature in the acute form of ADEM, which is also characterized by acute hemorrhagic leukoencephalitis, involves brain edema with numerous small hemorrhages. When cerebral edema is massive, this may cause temporal lobe and/or tonsillar herniation. Necrotic vascular lesions, focal demyelination and polynuclear infiltrates represent the main pathological findings; deposition of fibrin in vessel walls and in the Virchow-Robin spaces is a typical aspect.
ADEM usually develops one or two weeks following a viral disease or vaccination; its clinical presentation is characterized by fever, headache, vomiting and meningeal signs followed rapidly (in the most aggressive forms) by a combination of drowsiness, coma, seizures and focal, neurological signs. Recently, due to the progress of ICU care its prognosis has greatly improved. In a recent study about a cohort of 50 ADEM cases, an upper respiratory tract infection was evident in 50% of the patients.31 although transient cardiomyopathy and severe acute pulmonary edema have been reported in the acute stage.32
In the majority of ADEM cases, the cerebrospinal fluid shows only minor and unspecific changes. Cell count might be lightly increased accounting to the less than 10 cells/ml, with a total protein content also increased but usually below 100 mg/dl. In many instances, the immunoglobulin level is also elevated but oligoclonal bands are seen only rarely.33,34 In any case, when ADEM is suspected, it is very important to avoid dangerous delay as its differential diagnosis is quite difficult especially in the acute stage.
Magnetic resonance imaging can help in differentiating ADEM from multiple sclerosis although many features look the same, as it was in our case. In ADEM, lesions are typically extensive but poorly defined. They are mainly found in the white matter but can extend to the deep gray matter. Multiple sclerosis lesions are commonly found in the periventricular white matter and corpus callosum and are less likely to involve the gray matter.1-3 Patients with monophasic ADEM should not have new lesions on follow-up imaging, whereas MS may be a relapsing disease. Diagnosis of ADEM is usually retrospective; reviewing the clinical presentation along with laboratory and progression seen in neuroimaging. Prognosis for ADEM is favorable, although 20% of patients develop recurring demyelination and will eventually progress later on to frank multiple sclerosis.
We describe a dramatic presentation of ADEM in which accurate diagnosis was misleading which, we feel, may have led to suboptimal clinical management. Salvage, yet more than occasionally, life-saving procedure was attempted as extreme resource. This was not successful very likely because of acute development of irreversible lesions and massive refractory hemispheric edema. Although medical treatment alone is usually very effective in the management of patients with ADEM, one should bear in mind that in case of refractory brain edema and raised ICP, decompressive craniectomy may represent a lifesaving procedure. Even though in our case this was ineffective, we should not forget that in the international literature, there is anecdotal evidence suggesting the role of decompressive craniectomy as an effective and safe treatment.35-37
None.
None.

©2015 Houssem, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.