Journal of
eISSN: 2373-6410


Case Report Volume 11 Issue 6
1Department of Neurosurgery, Rutgers Biomedical and Health Sciences, USA
2Department of Neurosurgery, Clinic Las Condes, Chile
Correspondence: Allan J Drapkin, Alejandro Serani Norte 9458. Depto. 402,Vitacura. Santiago. Chile. Postal code 7640801, Tel +56233082519
Received: November 10, 2021 | Published: November 23, 2021
Citation: Drapkin AJ, Campos MP. Unilateral drainage of bilateral chronic subdural hematoma. J Neurol Stroke. 2021;11(6):155-158. DOI: 10.15406/jnsk.2021.11.00477
Bilateral chronic subdural hematoma (bCSDH) is a condition frequently encountered in neurosurgical practice, and it is usually the result of head trauma. Despite its frequency, no consensus currently exists regarding its optimal treatment. While the use of corticosteroids in the treatment of chronic subdural hematoma is not currently accepted by the neurosurgical community, there is enough evidence in the literature that supports its use. In bCSDH the unilateral burr hole evacuation of the larger of the subdural collections followed by a course of corticosteroids seems to be an effective and safer course of action in the management of bilateral chronic subdural hematoma.
Keywords: subdural hematoma, head trauma, corticosteroids, craniostomy
Unilateral chronic subdural hematoma (uCSDH) is a condition frequently encountered in neurosurgical practice, a frequency that fluctuates between 5.3 and 13.5 cases per 100.000 persons/year and increases in the elderly population.1,2 It will continue to grow because of the increasing age of the general population and the frequent use of anticoagulants and antiplatelet medications.
The currently accepted treatment for uCSDH is twist-drill or burr-hole craniostomy with evacuation of the subdural collection with or without irrigation of the subdural space and the elective placement of a closed-system subdural drainage.3 Despite its surgical simplicity, this procedure is not bereft of complications.4 bCSDH is also not rare, and presents a significant rate of complications and recurrences. Despite this situation, no well-defined treatment plan for it has been established to date, and its optimal management remains a matter of debate.5 Such a case is presented herewith with the treatment variation we implemented.
A review of the literature under the heading of chronic subdural hematoma was done and the reference lists of the publications thus gathered was reviewed in order to augment the data. Moreover, our case of a bilateral chronic subdural hematoma and its treatment is herewith presented.
Although the use of corticosteroids in the treatment of chronic subdural hematoma is not currently accepted by the neurosurgical community, there is enough evidence in the literature that supports its use.
An elderly patient, with a past medical history of well controlled hypertension, and a daily dose of 81 mg of Aspirin, experienced, for the three months preceding presentation, generalized headaches which gradually increased in frequency and intensity. In the last week the headaches became associated with memory disturbances and mental confusion which prompted her consultation. Her physical examination detected no obvious neurological deficits but an emergency CT scan revealed a bilateral chronic subdural hematoma (Figure 1A), larger on the right side with midline shift (Figure 1B) for which she was admitted.
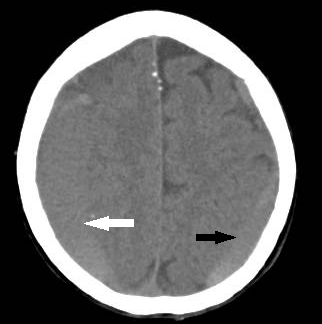
Figure 1A Non-contrasted CT scan showing a large, mostly isodense, right sided chronic subdural hematoma (white arrow) and a smaller left subdural collection (black arrow).
Routine blood tests, a chest x-ray and an EKG revealed no significant abnormalities. The patient was subsequently brought to the operating room where, under general endotracheal anesthesia and in the supine position with some head elevation, burr-holes were made in the right posterior frontal and parietal areas overlying the largest of the subdural collections. The dura was opened in a cruciform fashion at both sites and each one of the resulting dural quadrants was shrunk by bipolar coagulation so as to prevent the creation of a ball-valve mechanism that could interfere with the egress of the subdural collection into the subgaleal space where it could be absorbed. Moreover, the subdural space was irrigated with physiological solution but no subdural drain was placed. The patient tolerated the procedure well. The following day a repeat CT scan was done to assess the postoperative condition (Figure 2). She was then started on a ten-day course of Prednisone 5 mg PO QID and she was discharged. A repeat CT scan was done three weeks later (Figure 3).
The recognized cause of CSDH is usually a trivial head trauma that causes acceleration/deceleration of the intracranial content resulting in a traumatic cleavage between the inner layer of the duramater and the arachnoid thus transforming a virtual space into an actual subdural space.6 The simultaneous tear of the bridging veins in that area causes bleeding into that subdural space, a mechanism whose anatomical basis has been clearly demonstrated.7 The symptomatic expression of this subdural hematoma usually lags by weeks or months and, because the original trauma is usually a minor one, by the time of presentation it is frequently forgotten by the patients, a fact that explains the 30-50% of cases lacking such historical data upon admission.2
Blood within the subdural space incite an inflammatory reaction with deposition of fibrin and the eventual formation of subdural membranes that encapsulates the subdural blood. These neo-membranes, particularly the outer one, possess high levels of plasminogen and plasminogen activator8 and several angiogenesis-promoting factors, such as the vascular endothelial growth factor (VEGF), which induces the formation, in these neo membranes, of immature and leaky blood vessels with large gap junctions and weak basement membranes.9 The high content of plasminogen and plasminogen activator existing in the neomembranes, easily diffuses into the subdural collection, causing the enzymatic fibrinolysis and liquefaction of the subdural clot, resulting in a hyperfibrinolytic state with a high level of fibrin degradation products. This brings about the formation of defective clots that are easily lysed. These, together with the leaky blood vessels created in the outer neo membrane, result in frequent effusions of plasma and/or bleeding into the subdural collection leading to its gradual increase in size.10
The goal of any medical or surgical treatment for chronic subdural hematoma should be the complete resolution of the subdural collection, allowing brain re-expansion and the apposition of the outer subdural membrane onto the inner one, hypothetically causing scarring between these two membranes thus sealing the subdural space initially created and preventing the development of any further subdural collection, at least in the affected area. Confirmation of this hypothesis would not be easy to accomplish since it would require the pathological study of an autopsy series of difficult to collect cases that have recovered from CSDH but died from other causes.
Medical treatment of CSDH have been attempted with the use of Tranexamic acid, because of its antifibrinolytic activity,11 as well as with the angiotensin-converting enzyme (ACE) because of its capacity to inhibit angiogenesis.12 While the results obtained with these two pharmacological modalities have been encouraging, more experience with their use in this setting is needed before these could be incorporated into the CSDH treatment paradigm.
Corticosteroids have also been suggested and used for the treatment of CSDH in cases where anesthesia and surgery are not feasible,13 for cases with mild or moderate symptomatology,14 as an adjuvant to the surgical procedure16,17 and for CSDH too small to warrant surgery,16 while others have suggested its use as a first line of treatment for CSDH.17
While Dexamethasone´s therapeutic effect on CSDH is still debated,18 previous experience16,17 supports its use considering that corticosteroids actions interfere with the three main processes involved in the genesis and persistence of CSDH. Namely its anti-inflammatory activity,19 its inhibition of plasminogen activator thus reducing fibrinolysis20 and its inhibition of VEGF, thus decreasing angiogenesis,21 all actions that should promote CSDH resolution.
While the use of Dexamethasone in small CSDH is very effective without the need for surgical intervention,16 the sole use of Dexamethasone in large CSDH causing mass effect and midline shift, is not advisable because it would require a prolonged treatment with relative high doses of the drug that could result in significant side-effects. Furthermore, an emergent surgical evacuation could become necessary during this prolonged course of treatment.14,18 While the use of corticosteroids as the sole treatment of CSDH has demonstrated its capacity to reduce the rate of recurrences in CSDH, this type of treatment resulted in an increased rate of side-effects.18 This fact notwithstanding, the use of corticosteroids but as an adjuvant to the surgical evacuation of CSDH and using a lesser dose than the one used in that study has proven very effective and safe.16,17
bCSDH represent between 16 and 24% of the observed cases of CSDH.22,23 This bilateral form shows less lateralizing neurological deficits and less radiological midline shift than its unilateral counterpart, facts that may lead to a delay in its diagnosis.24 Moreover, bCSDH presents a reported 28% rate of recurrence, compared with the rate of recurrence in the uCSDH which is 9.59%.
Two recently published articles studying the results of unilateral surgical evacuation in bCSDH25,26 comprising a total of 394 cases stated that currently the decision to perform a unilateral evacuation on bCSDH is based on the relative volume of each subdural collection and in the presence of lateralizing clinical signs. The unilateral procedure in bCSDH was found in these studies, to be an independent risk factor for reoperation, a fact that led these authors to recommend the bilateral evacuation from the start. This recommendation cannot be taken lightly because, aside from requiring a longer time of anesthesia/sedation and increased intraoperative manipulation, the simultaneous evacuation of both CSDH in the geriatric population, where an associated brain atrophy may exist,27 could retard the postoperative brain expansion, and lead to intracranial hypotension with the potential development of tension pneumocephalus.28 An additional recent study analyzing this situation,29 established that the hypodense and the homogeneous CT densities in the preoperative non-operated subdural hematoma as well as the volume of the postoperative non-operated subdural collection, were important factors in predicting a postoperative contralateral hematoma progression, which could require a second surgical intervention.
The case presented herewith suggest a less invasive alternative by combining the unilateral evacuation of the larger subdural collection, causing a rapid improvement in the preoperative symptoms, followed by a ten days postoperative course of corticosteroids, which, by using a moderate dosage, should provoke no mayor side effects. The only additional requirement this regimen would impose is the close monitoring and control of the blood sugar level in those clinical or subclinical diabetic patients, for a period of time not significantly different than the usual period of postoperative follow up.
The potential risk inherent to the unilateral evacuation of a bCSDH, can be avoided with the postoperative use of corticosteroids which would promote the gradual and complete resolution of both subdural collections thus decreasing the expense and time involved in a more prolonged follow-up period with serial repeated CT scans. Obviously, the efficacy of this method should be confirmed by a multicenter randomized controlled study.
AJD contributed with the original conception of the work, reviewed the related literature, and wrote the first draft. MCP evaluated and operated the patient herewith presented, participated in the surgical planning, gathered the radiological material and critically reviewed the first draft. Both authors read the final version of the manuscript, approved its submission and are accountable for all aspects of the work.
No data sets were generated or analyzed for this study.
The authors wish to thank Paola Vermeer PhD, Ms. Camila Berner M.Sc. and Ms. Elizabeth Gonzalez for their bibliographical and technical assistance in the performance of this work.
The authors declare that they have no conflicts of interest.
Written informed consent was obtained from the individual prior to the publication of any potentially identifiable images or data included in this article.

©2021 Drapkin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.