Journal of
eISSN: 2373-6410


Research Article Volume 3 Issue 5
1Department of Neurology, 1Federal State-Funded Institution Russian Scientific Center for Medical Rehabilitation and Balneology, Russia
2University Clinic of Headache, Russia
3Department of Genetics, Lomonosov Moscow State University, Faculty of Biology, Russia
4Department of Neurology, Laboratory of Neurology and Clinical Neurophysiology, Research Center of the I.M. Sechenov First Moscow State Medical University, Russia
5University Diagnostic Laboratory, Russia
Correspondence: Eugene Klimov, Department of Genetics, Biological Faculty of Lomonosov Moscow State University, 119234, Moscow, Lenin Hills, 1-12, Russia
Received: November 28, 2015 | Published: December 9, 2015
Citation: Asimova J, Kondratieva N, Sergeev A, Skorobogatykh K, Kochetkova T, et al. (2015) The Role of Polymorphism of Regulatory Region of MTDH Gene (Rs1835740) in Migraine and Other Forms of Primary Headaches. J Neurol Stroke 3(4): 00101. DOI: 10.15406/jnsk.2015.03.00101
Objective: There is evidence that MTDH gene has a role in migraine pathophysiology. In our research, association of SNP in MTDH gene (rs1835740) with clinical parameters of migraine is considered.
Background: As a result of the first genome-wide association study (GWAS) of a common migraine, a SNP in the regulatory region of MTDH gene (rs1835740) was found. However, the confirmation of GWAS findings on independent samples failed. Also, there is no clear answer on the role of this substitution in the formation of the clinical picture of the various forms of migraine.
Patients and methods: The study included 143 patients with migraine. Comparison groups consisted of 9 patients with cluster headache and 20 patients with chronic tension headache. The control group included 362 unexaminated subjects. Genotypes were determined using real-time PCR with allelic discrimination test.
Results: Our study evaluated the role of rs1835740 substitution in the clinical picture of the various forms of migraine (episodic, chronic), as well as identified the specificity of this marker for migraine compared with other forms of headache (cluster headache, chronic tension headache). We have not found any effect of T allele (rs1835740) on the formation of the clinical picture of migraine with aura and migraine without aura. Also, we have shown that rs1835740 polymorphism has no effect on the chronification of migraine. Meanwhile, the carriage of T allele is specific for patients with migraine and cluster headache, but is not a characteristic feature of patients with chronic tension headache.
Conclusions: Our results suggest that the T allele of substitution rs1835740 in MTDH gene have not effect on the formation of the clinical picture of migraine with aura and migraine without aura, but specific for patients with migraine and cluster headache.
Keywords: MTDH, rs1835740, Migraine, Aura, Chronic Migraine, Cluster Headache, Chronic Tension Headache
MTDH, Metadherin; PGCP, Carboxypeptidase Q (CPQ gene); SLC1A2, Solute Carrier family 1 (glial high affinity glutamate transporter), member 2 (EAAT2); CSD, Cortical Spreading Depression
Genetic predisposition to migraine is well-known and proven by epidemiological genetic studies.1 The study of the genome in families of patients with hemiplegic migraine revealed five types of migraine with monogenic inheritance (familial hemiplegic migraine I, II, III, IV, V). However, these forms are extremely rare and are not involved in the development of common migraine with aura or without aura.2 The first genome-wide study (GWAS) of common migraine was published in 2010.3 This study included nearly 6,000 patients with migraine with or without aura from various European countries (the Netherlands, Denmark, Finland, Germany, Iceland) and more than 40,000 healthy individuals. Investigation revealed that the presence of minor T allele of rs1835740 substitution (NC_000008.11:g.97154685T>C) in 8q22.1 locus increases the risk of migraine, particularly migraine with aura. However, a case-control study conducted in Spanish population, including 1521 migraine patients and 1379 healthy subjects, did not reveal any significant differences in rs1835740 allele frequencies.4
The SNP rs1835740 is located between MTDH (metadherin) and PGCP genes that are involved in glutamate metabolism. Quantitative analysis of transcription activity of the MTDH gene in lymphoblastoid cell lines demonstrated that the expression level had a significant correlation with this substitution, since the minor T variant was associated with the high transcriptional activity of the gene.3 Earlier studies showed that MTDH negatively regulates the expression level of SLC1A2 gene (also known as EEAT1) encoding the major glutamate transporter protein.5 Presence of T allele of rs1835740 results in the accumulation of extracellular glutamate activating NMDA receptors involved in central sensitization, thereby reducing the neuronal excitability threshold.6 In addition, this substitution is located not far from PGCP gene encoding glutamate carboxypeptidase, an activator of astrocytes activity, and, therefore, a reduction occurs in the development threshold of cortical spreading depression, the underlying pathophysiological correlate of migraine aura.3 The pathophysiological association of rs1835740 polymorphism with the development of migraine confirms the fact that a mutation in ЕЕАТ1 gene results in the development of familial hemiplegic migraine.7
It can be assumed that rs1835740 polymorphism causes more severe migraine. The study by Esserlind et al8 that included 691 patients with migraine with aura investigated the details of clinical manifestations of migraine depending on genotype (42% were T allele carriers). T-allele carriers had a tendency to a greater representation of symptoms during aura, as well as to a lesser severity of headaches and representation of related symptoms. In TT homozygotes, these clinical patterns were not more pronounced. The authors concluded that T-allele of rs1835740 substitution increased the risk of migraine development, but had no effect on the formation of symptoms of migraine with aura.
In the study by Christensen et al.9 a phenotypic analysis of rs1835740 T-allele carriers in patients with migraine without aura was conducted. It included 339 patients, with 40% being carriers of T-allele. There were no significant differences in the representation of the migraine characteristics and symptoms, presence of comorbidities, representation of migraine attack triggers, effects of hormonal changes (pregnancy, COCs, postmenopause) on the frequency of migraine attacks as well as the effectiveness of triptans and preventive therapy.
These studies do not give a definite response about the role of rs1835740 polymorphism in the clinical picture of the various forms of migraine. The objective of our study was to determine the effect of a single nucleotide polymorphism rs1835740 in the clinical picture of the various forms of migraine (episodic, chronic), as well as identify the specificity of this marker for migraine compared with other forms of headache (cluster headache, chronic tension headache).
Patients
The study included 143 patients with migraine, with the average age of 41.6±12.5 years old (67.8% with episodic migraine, 32.2% with chronic migraine, 18.5% with migraine with aura, 31.9% abused painkillers). Comparison groups consisted of 9 patients with cluster headache and 20 patients with chronic tension headache. The control group included 362 unexaminated subjects. Patients from the main group, comparison group and controls were age-matched. Headache forms were diagnosed based on the criteria of the International Classification of Headaches III.10 Patients underwent a clinical neurological examination and blood sampling for genotyping. The study was approved by the local ethics committee and all subjects gave informed consent to participate in the study.
Molecular genetic testing and data analysis
The DNA extraction was performed according to the protocol for a commercial set DNA MagnaTM DNA Prep 200 (Isogene Laboratory LLC, Moscow). The assessment of allelic states of SNP studied was performed using real-time PCR. The primers, fluorescent probes to rs1835740, and PCR conditions were selected by DNA Synthesis, LLC (Moscow, Russia): F: CTGACGAATATACTTATATTCCTTTTACAT, R: CTTGCATATTTGAGCAGACTTTG, rs1835740-C: FAM-CCAATCTGCGTATGTAGA-BHQ2, rs1835740-T: VIC-CAATCTGTGTATGTAG-BHQ2. For real-time PCR a commercial kit qPCR mix (EvrogenJSC, Moscow) was used. PCR was performed on CFX96 (BioRad, US) using an allelic discrimination test. PCR conditions: 95 °C — 3', 40 cycles of 95 °C — 30'', 57 °C — 60'', 72 °C — 30''.
Statistical processing was performed using parametric and nonparametric methods available in software package IBM SPSS Statistics 22. Compliance with Hardy-Weinberg equilibrium and association with the disease in general were calculated using Pearson's chi-squared test (chi-square).
The frequencies of genotypes and alleles of rs1835740 substitution in patients with migraine and in the control sample are presented in Table 1. The genotype frequencies do not comply with Hardy-Weinberg equilibrium. Therefore, we used the multiplicative model (allele frequencies) to assess the association with the disease. No significant differences between patients with migraine and healthy subjects were obtained (χ2 = 0.24, p = 0.63). In general, the distribution of genotypes corresponds to that obtained by other authors.3,8
Frequencies of genotypes |
Frequencies of alleles |
||||
CC |
CT |
TT |
C |
T |
|
Patients |
0.788 |
0.178 |
0.034 |
0.877 |
0.123 |
HWE |
0.769 |
0.216 |
0.015 |
||
χ2=4.53; p=0.03 |
|||||
Control |
0.833 |
0.108 |
0.058 |
0.888 |
0.113 |
HWE |
0.788 |
0.2 |
0.013 |
||
χ2=75.35; p=0 |
|||||
Table 1 The frequencies of alleles and genotypes of the genes analyzed, compliance with Hardy-Weinberg equilibrium (df=1)
When comparing the representation of genotypes of rs1835740 in patients with migraine, cluster headache and chronic tension headache (Table 2), it was revealed that the T-allele carriage is not typical for patients with chronic tension headache, and the representation of the TT genotype is highest among patients with cluster headache.
Genotype |
СС |
СТ |
ТТ |
Migraine, n/% |
112/78.3** |
4/44.4 |
100/20 |
Cluster headache, n/% |
29/20.3 |
2/22.2 |
0/0 |
Chronic tension headache, n/% |
2/1.4* |
3/33.3 |
0/0 |
Table 2 rs1835740 genotypes in patients with migraine, cluster headache and chronic tension headache
* - Migraine vs. cluster headache, p = 0.001.
** - Migraine vs chronic tension headache, p = 0.0001.
The analysis of rs1835740 genotypes among different forms of migraine (migraine with aura, migraine without aura and chronic migraine) found no differences (Table 3).
Genotype |
СС |
СТ |
ТТ |
Episodic migraine (EM), % |
78.5 |
20.3 |
1.3 |
Chronic migraine (CM), % |
79.6 |
20.5 |
0 |
EM vs CM, p |
0.8 |
0.9 |
0.4 |
Migraine with aura (МА), % |
68.1 |
31.8 |
0 |
Migraine without aura (МбА), % |
82.1 |
16.8 |
1.2 |
MwO vs MwoO, p |
0.2 |
0.1 |
0.3 |
Table 3 rs1835740 genotypes in patients with different forms of migraine
Investigation of characteristics and symptoms of migraine in carriers and non-carriers of the T allele of rs1835740 showed no statistically significant differences in their representation (Table 4).
Symptom/Clinical Characteristic |
C-allele carriers |
T-allele carriers |
P-value |
Presence of migraine in relatives, % |
69.8% |
66.7% |
0.8 |
Age of migraine onset, years |
17.2±8.5 |
20.2±10.2 |
0.2 |
Duration of disease, years |
23.9±12.6 |
20.9±11.8 |
0.3 |
Frequency of migraine attacks per month |
9.0±10.6 |
8.2±8.3 |
0.7 |
Presence of aura, % |
16.1% |
29.2% |
0.1 |
Duration of attacks, hours |
34.6±25.2 |
36.5±34.1 |
0.8 |
Pain intensity, VAS scores |
8.3±1.5 |
8.5±1.2 |
0.4 |
Time to high intensity, minutes |
88.9±71.8 |
115.9±92.5 |
0.2 |
Pulsing pain |
78.9% |
79.2% |
0.9 |
Cutaneous allodynia, % |
51.2% |
34.8% |
0.1 |
Headache recurrence, % |
42.3% |
29.4% |
0.3 |
Nausea, % |
90.0% |
87.5% |
0.7 |
Vomiting, % |
45.5% |
50.0% |
0.7 |
Photophobia, % |
86.7% |
87.5% |
0.9 |
Phonophobia, % |
85.6% |
83.3% |
0.8 |
Osmophobia, % |
51.7% |
60.9% |
0.4 |
Presence of prodromal period, % |
31.7% |
50.0% |
0.1 |
Presence of postdromal period, % |
29.5% |
31.6% |
0.8 |
Resistance to standard therapy, % |
17.7% |
4.0% |
0.09 |
Presence of drug abuse |
36.7% |
26.9% |
0.3 |
Degree of drug abuse (1 point - mild, up to 30 individual doses of analgesics per month, 4 points - severe - more than 90 individual doses of analgesics per month) |
2.3 points |
2.1 points |
0.7 |
Table 4 Presence of symptoms and clinical characteristics of migraine among patients in carriers and non-carriers of T allele of rs1835740
Similar to previous studies performed by Christensen et al.9 and Esserlind et al.,8 we have not found any significant effect of carrying the minor T allele of rs1835740 substitution on the formation of the clinical picture of migraine with aura and migraine without aura. We have also shown that rs1835740 polymorphism had no significant effect on the development of chronic migraine.
Our study suggests that carriage of T allele of rs1835740 is specific for patients with migraine and cluster headache, but is not a characteristic feature of patients with chronic tension headache. Perhaps, impairment of glutamate homeostasis is a common step in the pathogenesis of migraine and cluster headache, ensuring the formation of cortical neuronal hyperexcitability.11,12 The effect of T allele on signaling pathways resulting in changes in the glutamate homeostasis and, consequently, cortical spreading depression (CSD) and aura is shown in Figure 1.
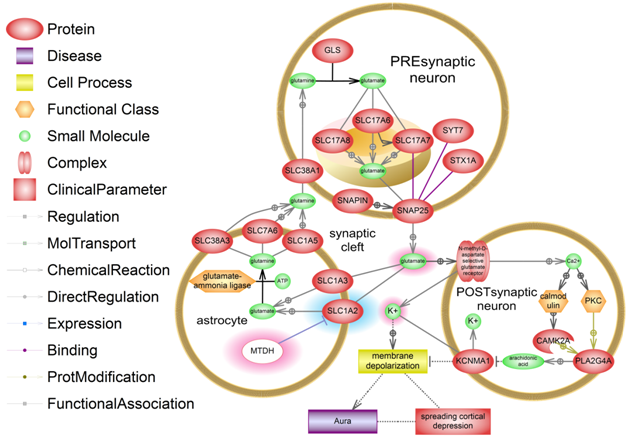
Figure 1 The effect of T-allele of rs1835740 SNP near MTDH gene on glutamate "overdose" in the synaptic cleft and CSD development. An abnormal increase and a decrease in the number of molecules are highlighted. MTDH protein is white. A detailed description is provided in the text.
MTDH protein is an expression blocker of one of the main of glutamate transporters from the synaptic space into an astrocyte, SLC1A2. In an astrocyte, glutamate is converted into glutamine and is transported into a presynaptic neuron, where it is converted back into glutamate. T allele of rs1835740 substitution causes increased MTDH gene transcription, which results in decreased expression of SLC1A2. As a result, glutamate accumulates in the synaptic cleft and continuously activates NMDA receptors on postsynaptic neurons, resulting in the release of potassium from the intracellular space onto the cell surface and the entry of calcium into the cell. Extracellular potassium causes the membrane depolarization. Hyperdepolarization underlies the CSD development -spreading depolarization of brain cells. The aura preceding a migraine attack is the result of CSD. A depolarization effect is enhanced by activation of phospholipase A (PLA2G4A) through the calcium-dependent pathways in the postsynaptic neuron. Phospholipase synthesizes arachidonic acid that blocks potassium channels and the potassium remains on the membrane surface.
The presence of cortical spreading depression and its clinical correlate, aura, is usually discussed in the context of neuronal hyperexcitability in migraine. However, up to 23% of patients with cluster headache can have a typical migraine aura prior to a headache attack,13,14 whereas the deficit of central analgesia systems largely underlies the development of chronic tension headache. Consequently, the T allele of rs1835740 causing an increase in extracellular glutamate levels provides for a CSD initiation and an attack onset. This assumption is confirmed by the findings of a genomic study by Anttila et al., which showed a greater impact of rs1835740 polymorphism on the development of migraine with aura.3
Thus, rs1835740 polymorphism is a clear risk factor for migraine; however, disease development and formation of clinical picture require the presence of additional extrinsic and intrinsic factors, including other genetic factors that need further study.
The authors thank the subjects for their participation in this research study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None.
Conception: Julia Azimova, Eugene Klimov, Andrey Rachin, GyusalTabeeva.
Clinical support: Julia Azimova, Alexey Sergeev, Kirill Skorobogatykh.
Molecular genetic analysis: Natalia Kondratieva, ZaremaKokaeva, TaisiaKochetkova, Eugene Klimov.
Statistical analysis: Julia Azimova, Eugene Klimov, Natalia Kondratieva.
Manuscript Preparation: Julia Azimova, Eugene Klimov.
Writing of the first draft: Julia Azimova, Eugene Klimov.

©2015 Asimova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.