Journal of
eISSN: 2373-6410


Case Report Volume 12 Issue 4
1Department of Neurology, Hospital de Braga, Portugal
2Department of Neuroradiology, Hospital de Braga, Portugal
Correspondence: Ana Rita Silva, Department of Neurology, Hospital de Braga, Braga, Portugal
Received: June 15, 2022 | Published: July 15, 2022
Citation: AR, Costa LR, Pinto J, et al. Orbital inflammatory syndrome after mechanical thrombectomy of acute carotid occlusion. J Neurol Stroke. 2022;12(4):90-93. DOI: 10.15406/jnsk.2022.12.00507
Introduction: Orbital ischemic syndrome (OIS) is a rare disorder that presents with acute loss of visual acuity, chemosis, proptosis, ptosis and ophtalmoparesis. We present a case with acute onset of orbital inflammation following mechanical thrombectomy that mimicked an OIS, except for the absence of vision loss.
Case report: A 65-year-old woman presented to our emergency department after an episode of transient dysarthria and left-sided hemiparesis and paresthesia that lasted about forty minutes. Head computed tomography showed no signs of intracranial bleeding or acute ischemia. CT angiogram showed occlusion of the right ICA terminus. Mechanical thrombectomy was then performed and the clot was removed with combined technique. Eight hours later, she developed orbital pain, ptosis, chemosis and complete ophthalmoplegia of the right eye. She had no diplopia, visual acuity and funduscopic examination were normal and there was no audible bruit in the orbit. Brain MRI performed showed hyperintensity of orbital fat along with hyperintensity and enlargement of all the extraocular There were no signs of orbital infarction and the optic nerve was structurally normal. Time of flight magnetic resonance angiography revealed reduced calibre of the distal portion of ICA, with associated contrast enhancement on vessel wall imaging. She was started on intravenous dexamethasone with full recovery two days later.
Discussion: We present the case of an orbital inflammatory syndrome after mechanical thrombectomy. The mechanical stress exerted by endovascular devices has been associated with endothelial damage that correlates with vessel wall thickening and contrast enhancement in high resolution MRI studies. The temporal correlation with mechanical thrombectomy, the absence of vision loss or orbital infarction and the good clinical response to steroids support the hypothesis of an internal carotid artery wall inflammation due to endovascular manipulation of the stent retriever to be responsible for this acute orbital syndrome. This case highlights the need for close postprocedural monitoring after MT and the consideration of orbital inflammation secondary to endovascular devices in the differential diagnosis of an acute orbital syndrome. Further studies are needed to understand the long-term consequences of arterial wall lesions associated with these devices.
Keywords: orbital inflammation, mechanical thrombectomy, vessel wall imaging, vessel wall thickening, endothelial damage
CCF, carotid-cavernous fistula; CT, computed tomography; CTA, computed tomography angiogram; ICA, internal carotid artery; MRI, magnetic resonance imagint; mRS, modified Rankin scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale ; OIS, orbital infarction syndrome
Orbital ischemic syndrome (OIS) is defined as ischemia of all intraorbital and intra-occular structures including the optic nerve, extraocular muscles and orbital fat.1 Firstly described by Vergez in 19592, it presents with acute loss of visual acuity, chemosis, proptosis, ptosis and ophtalmoparesis and results from hypoperfusion of the ophthalmic artery and its branches. This rare disorder, with less than one hundred cases reported in literature, has been described in relation to infectious and inflammatory diseases, nephrotic syndrome, arterial dissection, hypoperfusion from common carotid artery occlusion and invasive procedures, such as aneurism surgery or endovascular mechanical thrombectomy (MT).1–14
In 2015, five clinical trials showed the benefit of endovascular MT in acute large vessel occlusions.15 Since then, an increasing number of stroke patients are being treated with MT and more potentially adverse events have been recognized. Hereby, we present the case of a transient inflammatory orbital syndrome after MT that mimicked an orbital infarction syndrome.
A 65-year-old woman, with a pre-stroke modified Rankin scale (mRS) score of 0, was admitted to a tertiary hospital after an episode of transient dysarthria and left-sided hemiparesis and paresthesia that lasted about forty minutes. Her medical record included arterial hypertension, dyslipidemia and tension headache. One year ago, she had a transient ischemic attack with speech disturbance one year before. At that time, she was admitted to complete stroke workup, including brain magnetic resonance imaging (MRI), transthoracic echocardiogram, 24-hour Holter monitoring and carotid artery doppler ultrasound which were normal. She was discharged with aspirin 100mg, bisoprolol 5mg, simvastatin 20mg and amitriptyline 10mg.
On admission, she had no neurological deficits (National Institutes of Health Stroke Scale (NIHSS) 0). Head computed tomography (CT) showed no signs of intracranial bleeding or acute ischemia (Alberta Stroke Programme Early CT Score (ASPECTS) 10) (Figure 1A). CT angiogram (CTA) showed an acute right distal internal carotid artery (ICA) occlusion, without other significant occlusions or stenosis (Figure 1B). Our stroke team was contacted at this moment and the patient was transferred to our hospital, to proceed with MT, where she arrived nine hours after symptoms onset. Her NIHSS score was still 0. Her systolic blood pressure fluctuated between 170 and 200 mmHg and diastolic blood pressure ranged from 65 to 85 mmHg.
Following right femoral artery punction an 8F artery short sheath was placed. The right common carotid artery was then catheterized using a Neuron Max and a 6F Simmons II catheters. The first angiographic run confirmed an occlusion at the cavernous segment of the right ICA (Figure 1C). The clot was removed after a single pass with combined technique (using a 6,5 x 45 mm stentretriever and distal aspiration). After clot removal, a residual right M1 stenosis was seen. The final modified Thrombolysis in Cerebral Ischemia was 3 (Figure 1D). The femoral access was closed with a 6F Angio-Seal vascular closure device. At the end of the procedure, she had a normal neurological examination (NIHSS 0) and no orbital involvement was noticed. In the following hours, the systolic blood pressure ranged from 90 and 110 mmHg and the diastolic blood pressure between 45 and 55 mmHg.
Eight hours later, she complained of right orbital pain. Ptosis, chemosis and complete ophthalmoplegia of the right eye were observed (Figure 2 A-D). Visual acuity and funduscopic examination were normal and there was no audible bruit in the orbit. A repeat CT did not show any acute ischemic or hemorrhagic lesions. CTA showed a slight enlargement of the right medial and lateral rectus muscles. To rule out a possible carotid-cavernous fistula (CCF), cerebral angiography was performed and was negative for complications or fistulae. Steroid treatment ensued with IV dexamethasone (8 mg, three times a day). Brain MRI performed forty-eight hours after symptoms onset showed punctuate acute ischemic lesions in the right hemisphere (Figure 1E&F). Coronal T2 with fat saturation showed hyperintensity of orbital fat along with hyperintensity and enlargement of all extraocular muscles (Figure 1G). There were no signs of orbital infarction or cavernous sinus thrombosis. Optic nerve was structurally normal. Time of flight magnetic resonance angiography revealed calibre reduction of the distal portion of ICA (Figure 1I), with corresponding contrast enhancement on vessel wall imaging (Figure 1H).
Twenty-four hours later she had fully recovered (Figure 2E-H) and was discharged home one week later. Investigation for cardiac sources of embolism or thrombophilia was non-revealing. Steroid were slowly tapered for a month. At one month follow-up, she had resumed normal life (mRS 0).
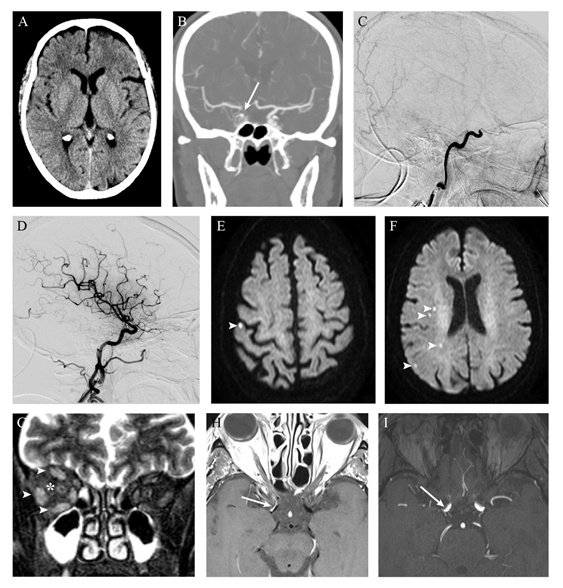
Figure 1 Radiologic findings. Early non-contrast CT excluded intracranial hemorrhage and showed no features of acute ischemia (A) A contrast filling defect on the terminal right internal carotid artery (arrow) was revealed on CT angiography. Digital subtraction angiography demonstrated occlusion of the right internal carotid artery at the cavernous segment (C) After mechanical thrombectomy, complete recanalization was obtained, with no signs of complications (namely carotid-cavernous fistula) (D) Brain MRI performed two days later showed only scarce foci of restricted diffusion (arrowheads) on DWI images (E-F), with corresponding low signal on apparent diffusion coefficient map (not shown), reflecting ischemic lesions on the vascular territory of right internal carotid artery. Coronal T2 fat satured images revealed right orbital inflammatory features manifested by faint hyperintensity of orbital fat (*) and extraocular muscles enlargement with hyperintensity (arrowheads) (G) Vessel wall imaging after contrast administration showed eccentric enhancement of the anterior wall of distal internal carotid artery on the right (arrow, H), where luminal stenosis was found on time-of-flight angiography (I).
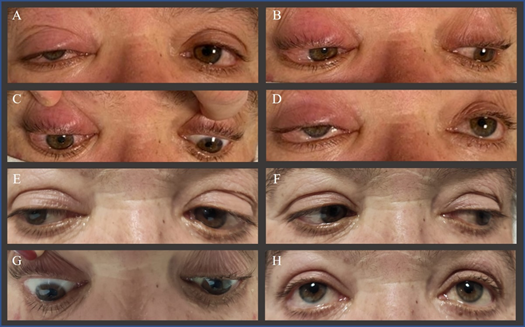
Figure 2 Eye movements eight hours after mechanical thrombectomy (A-D) and two days after starting intravenous dexamethasone (E-H). The right eye shows conjunctival chemosis and periorbital swelling (A-D) along with marked limitation of abduction (A) infraduction (C) and supraduction (D) and reduction of adduction (B) Two days later, ptosis, chemosis and ophthalmoplegia improved in all gaze positions (E-H).
We present a new case of an orbital inflammatory syndrome after MT of right ICA occlusion, with rapid recovery after a short course of steroid therapy.
MT is the standard of care in selected patients with ischemic stroke caused by large vessel occlusion with or without previous intravenous thrombolysis. Despite its demonstrated efficacy, MT is an invasive procedure that is not exempt from complications, ranging from 4% to 29% in randomized clinical trials.16 Complications can be classified in two categories: 1) intraprocedural complications, which can be further divided into access- and device-related difficulties and 2) postprocedural complications, mainly related with intracranial ischemic and haemorrhagic complications.17,18 Access site complications comprise femoral artery dissection, pseudoaneurysm, arteriovenous fistula, retroperitoneal hematoma, acute lower limb ischemia, neuropathies and site infection. Device-related complications include artery dissection, vasospasm, vessel perforation, stent detachment, carotid-cavernous fistula, embolization within the same or a new vascular territory and reocclusion. Symptomatic intracranial hemorrhage, subarachnoid hemorrhage, reperfusion injury and cerebral edema are the most common postprocedural complications.16–18
To achieve clot retrieval, MT can be performed with different techniques and similar efficacy and safety: mainly stent retrievers, aspiration catheters or a combination of both.15 Animal histopathological data have raised concern about the local effects of endovascular manipulation with these devices in intracranial vessel walls.19,20 This mechanical stress can lead to disruption of the internal elastic lamina and edema of the inner layers of the arterial wall, which in turn promote permanent damage with arteriosclerosis. These arterial changes have been correlated with vessel wall thickening and contrast enhancement in MRI studies with high-resolution vessel wall imaging within 3 days and 3 months after MT.21–26 The arterial wall thickening is explained by intima edema, whereas increased endothelial permeability to intravascular gadolinium would be responsible for the arterial wall enhancement.21 Gadolinium contrast enhancement was observed irrespective of the device used; however, Kasab and colleagues suggested that the use of stent retrievers is associated with a higher enhancement as compared to aspiration catheters.26 This arterial wall abnormality is less common among patients treated with medical therapy alone, suggesting that vessel wall enhancement is the result of the abrasive forces exerted by the devices rather than the thromboembolism itself.21,25
The extracranial ICA (C1 or cervical carotid) travels through the carotid sheath until entering the skull through the carotid canal. The intracranial ICA is then divided in six segments (C2-C6) and ultimately divided in two terminal branches: the anterior and middle cerebral arteries. The ophthalmic artery has its origin in the ophthalmic segment (C6) and passes through the optic canal to enter the orbit and supply its contents (the eyeball, the optic nerve, and the extraocular muscles). Although occlusion of the internal carotid artery is a common finding, ischemia of the orbital structures is extremely rare due to extensive anastomotic connections between the ophthalmic artery and branches from the external carotid artery.27
The acute onset of painful ptosis, chemosis and ophthalmoplegia after MT requests a differential diagnosis between OIS and carotid cavernous fistula (CCF). The absence of visual loss, a relative afferent pupillary defect and/or retinal ischemia on fundoscopy made the first hypothesis less likely. On the other hand, the absence of a bruit on orbital auscultation, engorgement of conjunctival and episcleral blood vessels and/or sensory impairment in the ophthalmic distribution of the trigeminal nerve were against the diagnosis of CCF. Diffusion weighting in MRI excluded orbital ischemia and a second cerebral angiography discarded the presence of CCF. At this point, the presence of orbital pain along with vessel wall thickening and contrast enhancement in vessel wall MRI argued in favour of an inflammatory process. The time elapsed between the endovascular procedure and symptom onset also supported an inflammatory rather than ischemic aetiology. She had no fever and infectious laboratory tests were normal which excluded infectious causes. Other causes of orbital inflammation could be considered but bearing in mind the temporal relationship with MT and the rapid clinical response after corticosteroids, we considered inflammation of the wall of the ICA due to endovascular device manipulation as the most likely diagnosis. The excellent outcome of our patient also contrasts with previous reports of permanent vision loss in OIS after MT.11,13
The arterial wall inflammatory process may be due to the direct effect of the mechanical forces exerted by the stent retriever, as well as the indirect effect of retrieval of the occluded thrombus that precipitates the release of inflammatory mediators. Previous studies have shown an increased level of inflammatory markers after an ischemic stroke.28–30 It is worth noting that the clot was occluded in the ICA for at least 10 hours. Corticosteroids have been shown to be effective in reducing both vascular and extravascular edema in orbital diseases.7,31,32 Ongoing research has also proposed that endothelial injury following mechanical thrombectomy may impact the patient outcome by compromising the blood-brain barrier, initiating an inflammatory cascade and compromising downstream cerebral autoregulation.29,30 With the growing number of endovascular procedures, these inflammatory complications may become more commonly reported.
The incomplete orbital syndrome of our patient, without clinical or imagiologic evidence of orbital ischemia, along with major improvement after two days of cortical therapy suggested a transient inflammatory orbital syndrome precipitated by stent retriever MT. Important teaching points of our case include: 1) close postprocedural monitoring after MT and the consideration of orbital inflammation secondary to endovascular devices in the differential diagnosis of an acute orbital syndrome, 2) a multidisciplinary approach is essential to better recognize and manage potentially iatrogenic endovascular events and 3) further studies are needed to understand the long-term consequences of arterial wall lesions associated with these devices.
None.
None.

©2022 AR,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.