Journal of
eISSN: 2373-6410


Case Report Volume 2 Issue 6
1Burzynski Clinic, Houston, TX, USA
2CHIP ADVANCED Hospital Group, SPAIN
Correspondence: Stanislaw R Burzynski, MD, PhD, Burzynski Clinic, 9432 Katy Freeway, Houston, TX 7755, USA, Tel 713-335-5697, Fax 713-935-0649
Received: May 30, 2015 | Published: September 10, 2015
Citation: Burzynski SR, Burzynski GS, Marszalek A, et al. Long-Term survival over 21 years and pathologically confirmed complete response in pediatric anaplastic astrocytoma: a case report. J Neurol Stroke. 2015;2(6):1-6. DOI: 10.15406/jnsk.2015.02.00072
Inoperable pediatric anaplastic astrocytoma (PAA) is a rare, malignant, brain tumor and is not curable with current treatments. A 12-year-old female with newly-diagnosed PAA presented with a 4.8x2.1 cm tumor involving the left temporal lobe, crossing the midline and compressing the pons. In November 1993 the patient underwent a craniotomy and biopsy of the tumor, but did not have radiation or chemotherapy. Approximately one month later, she presented to Burzynski Clinic (BC) and was treated with antineoplaston (ANP) A10 and AS2-1 injections based on protocol CAN-01. The treatment was given at the average dosage of 3.4 g/kg/d of A10 and 0.4 g/kg/d of AS2-1. All symptoms, except seizures, disappeared by 13 months. Magnetic resonance imaging (MRI) of the head at 32 months showed a complete response. After the 40th month, the patient was switched to A10 and AS2-1 capsules (0.14 g/kg/d each) until the 56th month. The time was measured from the treatment start. At that time she underwent resection of the tissue causing the seizures. The pathological examination did not demonstrate any presence of the neoplastic process. The follow-up MRIs between 6 and 15 years since the treatment start did not show tumor recurrence. She continues to live a normal life over 21 years later.
Keywords:Antineoplastons A10 and AS2-1, Brain tumor, High-grade glioma, Pediatric anaplastic astrocytoma, Progression-free survival
AA, Anaplastic Astrocytoma; ANP, Antineoplastons A10 and AS2-1; BC, Burzynski Clinic; BRI, Burzynski Research Institute, Inc.; CR, Complete Response; CTCAE v.3, Common Terminology Criteria for Adverse Events, version 3.0; DIPG, Diffuse Intrinsic Pontine Glioma; FDA, Federal Drug Administration; HGG, High-Grade Glioma; IND, Investigational New Drug; IRB, Institutional Review Board; isoPG, Phenylacetylisoglutaminate Sodium; KPS, Karnofsky Performance Status; MRI, Magnetic Resonance Imaging; OS, Overall Survival; PAA, Pediatric Anaplastic Astrocytoma; PFS, Progression-Free Survival; PG, Phenylacetylglutaminate Sodium; PN, Phenylacetate Sodium; PR, Partial Response; SD, Stable Disease; TMZ, Temozolomide
Pediatric anaplastic astrocytoma (PAA) (in children from 10 to 14 years of age) is a rare tumor. The average annual incidence rate in the United States is 92 cases out of a total of 4,750 cases of pediatric tumors of neuroepithelial tissue, which constitutes of less than 2%.1 Clinical trials were not performed for PAA, but patients with this diagnosis were grouped together with children with other high-grade gliomas (HGG).2,3 The prognosis for pediatric HGG is generally poor. There is a low percentage of long-term survival and most of the patients die from their disease.2 Lashford et al.4 reported data on the treatment of 33 patients diagnosed with HGG using radiation therapy and temozolomide (TMZ); the median survival was 4.7 months.4 It is generally considered that the extent of surgical resection is an important prognostic factor, but in children with tumors inaccessible for surgery, the prognosis is worse.5 However, certain chemical therapeutics have been used as alternative treatments for pediatric HGG when surgical resection is not safe. Specifically, Antineoplastons (ANP) A10 and AS2-1 injections are synthetic amino acid derivatives that have been utilized in this manner. A10 is a formulation consisting of a 4:1 ratio of phenylacetylglutaminate sodium (PG) and phenylacetylisoglutaminate sodium (isoPG). AS2-1 is a formulation with a 4:1 ratio of phenylacetate sodium (PN) and PG. ANP A10 capsules contain 500 mg of 3-phenylacetylamino-2,6-piperidinedione, which is metabolized in the body to 4:1 ratio of PG, and isoPG and AS2-1 capsules contain 500 mg of 4:1 ratio of PN and PG.6 Interim results of the phase II studies have already been reported as well as case reports of long-term survival of HGG and diffuse intrinsic pontine glioma (DIPG).3,7-16 Here, we discuss a case of very long survival (over 21 years) of PAA with pathologically-confirmed complete response (CR).
A 12-year-old Caucasian female patient in prior good health and with no history of neurofibromatosis 1 (NFM1) presented to an outside medical center. Head magnetic resonance imaging (MRI) in November 1993 revealed a large tumor with contrast-enhancement located in the left temporal lobe crossing the midline and compressing the superior aspect of the pons. Three weeks later, the patient underwent craniotomy and biopsy of the left medial temporal mass which showed a pleomorphism of the tumor cells and presence of definite mitoses. Following a consultation with several pathologists and neuropathologists, a diagnosis of anaplastic astrocytoma (AA) was made. She did not have any further treatment. Repeated MRIs at 5 and 9 weeks from initial MRI showed increasing size of the tumor.
In the first week of January, 1994, the patient presented to Burzynski Clinic (BC) with complaints of headaches, sensation of pressure in the head, occasional nausea, decreased memory, slurred speech, increased tiredness and sleepiness, focal epileptic seizures (occurring approximately four times per day), and occasional grand mal seizures. On physical examination, the patient was slow to respond and had slurred speech, but otherwise had a negative examination. There were no neurocutaneous lesions. The Karnofsky Performance Status (KPS) was 60. Head MRI demonstrated an infiltrating, enhancing tumor involving a large portion of the left temporal lobe, crossing the midline, and compressing the brainstem. Pediatric AA may not have post-contrast-enhancement, but the enhancing AA lesions also have been described in this age group.2,9,10 The two largest perpendicular diameters of the contrast enhancing lesion were 4.8x2.1 cm. Treatment with intravenous ANP A10 and AS2-1 was started on January 7, 1994 at the dosage of 0.10 g/kg/day for both A10 and AS2-1, and then escalated to 3.43 g/kg/day and 0.46 g/kg/day, respectively. The details are provided in Table 1. The treatment was according to Protocol CAN-1.6
Place and Date |
Diagnosis/Response |
Treatment |
University Hospital, Radiology MRI of the Head November 1993* |
High-grade glial tumor of the medial temporal lobe |
|
University Hospital, Neurosurgery November 1993 |
Craniotomy, left temporal biopsy, left medial temporal tumor |
|
University Hospital, Pathology November 1993 |
Astrocytoma, Grade 3 (anaplastic) |
|
University Hospital, Radiology MRI of the Head December 1993 |
Increase of tumor size |
|
University Hospital, Pathology January 1994 |
Astrocytoma, Grade 1 |
|
Regional Medical Center, Radiology MRI of the Head January 10, 1994 |
Increase of tumor size |
|
University Hospital, Pathology Consultation with several pathologists and neuropathologists January 1994 |
Astrocytoma, Grade 3 (anaplastic) |
|
Burzynski Clinic January 7, 1994 to December 18, 1995 |
Stable disease |
ANP I.V. |
Burzynski Clinic December 19, 1995 to September 26, 1996 |
Partial Response |
ANP I.V. |
Burzynski Clinic September 27, 1996 to May 26, 1997 |
Complete Response |
ANP I.V. |
Burzynski Clinic May 27, 1997 to September 11, 1998 |
Continuation of complete response |
ANP p.o. |
University Hospital, Neurosurgery November 1998 |
Left temporal lobectomy and biopsy of the left temporal lobe |
|
University Hospital, Pathology November 1998 |
Gliosis and reactive astrocytes. No evidence of residual tumor |
|
Regional, Radiology MRI of the Head February 11, 1999 to June 10, 2009 |
Complete Response |
Table 1 Summary of diagnosis and treatment. Abbreviations: ANP: Antineoplastons therapy, I.V. - Intravenous, P.O. - By mouth. *Exact dates can be identifying, and they are not included in this report
Follow-up brain MRIs at 3 and 14 months from treatment start did not show significant changes. However, all of the patient’s symptoms except epileptic seizures disappeared by 13 months. Head MRIs at 23 and 29 months of treatment showed the corresponding two largest perpendicular diameters of the contrast enhancing lesion to be approximately 1.3x0.3 cm and 1.0x0.2 cm, respectively, indicating a 96% and 98% decrease, respectively, compared to the MRI at the time of the patient’s presentation to our clinic. Further, follow-up MRI at 32 months after presentation to our clinic showed complete disappearance of the enhancing lesion, versus questionable minimal enhancement indicating the beginning of a CR. At this point, the patient’s mild seizures occurred approximately once per month, were associated with her menstrual cycle, and were suspected to be triggered by a scarred area of brain which was residual to the CR.
Based on a similar follow-up MRI at 37 months after presentation, treatment with intravenous ANP was discontinued at 40 months, and the patient continued maintenance treatment with ANP A10 and AS2-1 capsules at a dosage of 0.14 g/kg/day each. Follow-up MRIs at 45, 51 and 55 months after presentation to our clinic confirmed CR and antineoplaston treatment was discontinued at 56 months. At 58 months, the patient underwent resection of gliosis scar causing seizures. Pathologic evaluation of the biopsy did not demonstrate any presence of astrocytoma, and the concluding diagnosis was mild grade cerebral cortical dysplasia representing a glioneuronal hamartoma. The patient recovered well from surgery and has not had any epileptic seizures since then. The details of her diagnosis and treatment are shown in Table 1. Follow-up MRIs, shown in Figure 1, between 6 and 15 years after presentation to our clinic have not shown any tumor recurrence. Importantly, the patient did not report any serious adverse events due to antineoplastons.
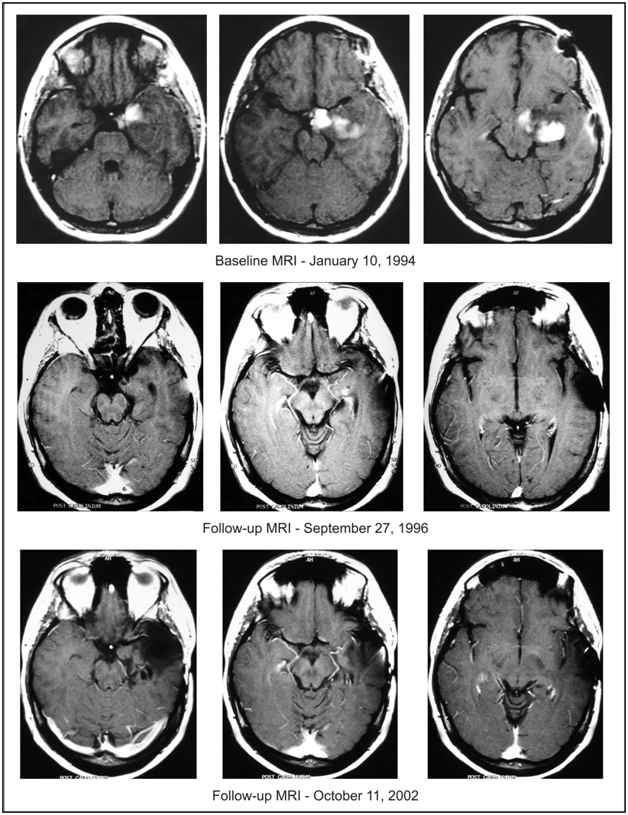
Figure 1 Baseline MRI of the head with contrast. Follow-up MRI of the head with contrast on September 27, 1996, indicating complete response. Follow-up MRI of the head with contrast on October 11, 2002, after resection of gliosis scar, indicating continuation of complete response.
Additional drugs administered to the patient included dexamethasone (Table 1), benzodiazepine, vigabatrin, gabapentin, furosemide, triamterene, hydrochlorothiazide, ranitidine, cephalexin, and morphine sulfate. Dexamethasone was started before treatment with ANP and was tapered-down and discontinued on January 28, 1995. It was prescribed again by the local physician from January 10, 1996 to January 26, 1996, from April 25, 1996 to May 4, 1996, and from February 19, 1997 to March 15, 1997, when she developed increased seizure activity. The patient is now over 21 years since her treatment start, continues to do very well, and lives a normal life. Her progression-free survival (PFS) is over 21 years.
The patient was treated in Phase II clinical studies under the FDA’s Investigational New Drug Application (IND 43,742) and was monitored by an independent institutional review board (BRI IRB). Pathology diagnosis and responses to the treatment were evaluated and confirmed by outside pathologists and radiologists. The adverse events were graded according to the Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE v.3).
We have presented here an interesting case of PAA who accomplished a CR, confirmed radiologically and pathologically, and an overall survival in excess of 21 years. Recent clinical trials in pediatric HGG were reviewed extensively.2,3,9,10 Unfortunately, the results of these studies show that the efficacy did not improve beyond standard radiation therapy, which still remains the accepted treatment. The overall results indicate that less than 10% of children with HGG and 40% of children with PAA in this age group do not survive more than two years from diagnosis, and 29% with PAA live beyond five years.1 Less than ten trials have been conducted recently in a mixed population of pediatric and adult patients. Typically these trials accepted patients with recurrent HGG and also patients with DIPG. They will not be discussed here since they have been reviewed before.2,3,9,10
In terms of newly diagnosed HGG, the results of a multicenter phase II study by Gilbert et al.17 of temozolomide (TMZ) were published.17 Fifty-seven patients (51 adult and 6 pediatric) were accrued to the trial. Here, 18 patients had the diagnosis of anaplastic astrocytoma, and all such patients had a KPS between 90 and 100. Initial treatment consisted of TMZ 200 mg/m2/day for 5 consecutive days, every 28 days, for a maximum of four cycles. Subsequently, all patients were treated with standard external beam radiation therapy. In the AA patients, 10% achieved CR, 24% partial response (PR), and 38% had stable disease (SD). Median PFS was 7.6 months, and median overall survival (OS) was 23.5 months. The OS probability at 12 and 24 months was 70% and 50%, respectively. In the total patient population, grade 3 and 4 adverse events were reported in 28% and 12%, respectively. The conclusion of this study was that TMZ was safe and effective in treating newly diagnosed AA before radiotherapy. Such a treatment approach appears promising, but requires additional evaluation and comparative studies.
The results of three phase II studies of ANP A10 and AS2-1 in children with HGG have been published already, but the data about the treatment of this patient has not yet been reported.8-10 The mechanism of action of ANP has been described in detail before.18,19 The patient described in this article is surviving over 21 years since the treatment start and lives a normal life. There are no chronic adverse events and her quality of life is normal for her age.
In conclusion, we have presented a case of PAA with unusually long-term OS and PFS of over 21 years. The only treatment the patient received were intravenous and oral ANP without surgical resection, radiation therapy or chemotherapy. There were no significant acute adverse events and no chronic toxicity, and quality of life was excellent. This patient accomplished CR on intravenous ANP. The contributions of oral ANP can be evaluated by an additional clinical study.
The authors express their appreciation to the additional physicians involved in the care of the patient: Robert A. Weaver, M.D., Eva Kubove, M.D., Barbara Szymkowski, M.D., and Mohammad Khan, M.D., and employees of the clinic involved in the preparation of the manuscript: Carolyn Powers and Adam Golunski.
All authors, except for Dr. Juan F. Martinez-Canca, are employed by Burzynski Clinic. Dr. Stanislaw R. Burzynski (SRB) is President at the Burzynski Clinic. The clinical trial was sponsored by the Burzynski Research Institute, Inc., (BRI). SRB is Chairman of the Board of Directors and President of BRI Inc. Dr. Gregory S. Burzynski is Vice President of Burzynski Clinic and a member of the Board of Directors of BRI Inc. Dr. Tomasz J. Janicki is the Vice President of Clinical Trials at BRI Inc. Dr. Juan F. Martinez-Canca is a neurosurgeon in Spain.

©2015 Burzynski, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.