Journal of
eISSN: 2373-6410


Review Article Volume 12 Issue 4
1Neurosurgery Resident University of Cartagena, Colombia
2Neurosurgeon Hospital Universitario del Caribe, Colombia
3Primary Care Physician in Epilepsy and Neurological Sciences. Jaime Fandiño Franky Colombian Center for Epilepsy and Neurological Sciences Foundation, Colombia
Correspondence: Claudio A Rivas Palacios, Neurosurgery Resident University of Cartagena (Colombia)
Received: July 27, 2022 | Published: August 23, 2022
Citation: Palacios CAR, Miranda HRA, Pacheco MAE. Hydrocephalus secondary to tumors of the posterior fossa: review article. J Neurol Stroke. 2022;12(4):124-129. DOI: 10.15406/jnsk.2022.12.00514
Posterior fossa (PF) tumors are a common cause of obstructive hydrocephalus. In adults, metastases are the most common tumors in PF. Among the primary neoplasms, the most common are meningioma, vestibular schwannoma, and hemangioblastoma. Glial tumors are the most prevalent in children and adolescents, followed by medulloblastoma and ependymomas.
Surgical treatments are of choice in hydrocephalus due to posterior fossa tumors (HPFt). Tumor resection may be sufficient as monotherapy in adults with asymptomatic hydrocephalus and in others with a low risk of postoperative hydrocephalus. Endoscopic third ventriculoscisternostomy (ETV) is the choice for prophylaxis and / or treatment of HPFt in children-adolescents and adults. Ventriculoperitoneal bypass is beneficial in children-adolescents and adults with relative contraindications for EVT and / or unfavorable anatomy for it. External ventriculostomy is useful in adults who require emergency cerebrospinal fluid drainage.
The posterior fossa (PF) is an anatomical region susceptible to complications. Its location, size and content of vital structures mean that any change in its content could put the patient's life at risk. In children and adolescents, PF tumors account for 60% of all brain neoplasms, while in adults they account for 20%.1 One of the main complications associated with localized tumors in PF is hydrocephalus.2,3 This has high morbidity and mortality, and therefore great economic impact due to the resources of the health system invested in its treatment. HPFt accounts for approximately 10% of all hydrocephalus in children and adults (9% and 11%, respectively).4,5 HPFt occurs in children and adolescents up to 90%,6–8 and in adults,9,10 are reported in up to 58% of cases. Despite its high frequency, there is still controversy regarding the optimal moment and the treatment modality. This review article aims to describe the pathophysiological mechanisms and risk factors involved in the development and/or perpetuation of HPFt, as well as the different therapeutic approaches and treatments, establishing its main indications.
Hydrocephalus is an active dilatation of the ventricular system of the brain, resulting from the inadequate passage of CSF from its place of production within these ventricles, to its site of absorption in the systemic circulation.11
From the pathophysiological point of view, hydrocephalus is classified as obstructive and non-obstructive. Hydrocephalus is considered to be obstructive when there is partial or total blockage of CSF circulation through the foramina (Monro, Luschka and Magendie) and ducts (cerebral aqueduct) that connect the ventricles with each other, and finally with the ventricles. subarachnoid space. In the case of non-obstructive hydrocephalus, it can be due to an increase in the production of CSF in the choroid plexuses (eg: choroid plexus tumors), or due to a decrease in its absorption in the arachnoid granulations (mainly). HPFt is predominantly obstructive.12–14 It is classified as intra-axial if the tumor lesion exerts a mass effect on the outlet of the fourth ventricle (V4), and extra-axial if what occurs is a secondary compression of the venous outflow.6,12
The severity of hydrocephalus can be established with the standardized method used by Choudhury in 1995,15 determining the ventricular/biparietal (V/BP) ratio (mathematical ratio) from a computed tomography of the skull (axial slice). The V/BP ratio is calculated from the measurements of the ventricular diameter (parietal part of the body of the ventricles, drawing a line between the lateral walls of both lateral ventricles) and the biparietal diameter (on the same "ventricular line" is extended to the internal cortices of the skull bilaterally) (Figure 1). The V/BP is considered normal when it is less than 0.26. Taking these variables into account, 4 severity groups are established: mild (V/BP 0.26-0.40), moderate (V/BP 0.41-0.60), severe (V/BP 0.61- 0.90) and extreme (V/BP 0.91-1).15
CSF is one of the three main elements contained in the skull and contributes 10% of the intracranial volumen.16–20 The total capacity of the cerebrospinal cavity in the adult is 1,400 to 1,700 ml. The volume of CSF varies from 75 to 270 ml in the adult divided between the ventricular system (25%), spinal canal (20 to 50%) and subarachnoid space (25 to 55%).17
CSF is an ultrafiltrate of plasma. It is produced in 60-70% in the choroid plexuses of the lateral ventricles (LV), third ventricle (V3) and fourth ventricle (V4). The remaining 30% of production is extrachoroidal, with the participation of the ependyma, the arachnoid and subarachnoid epithelial membrane, and the neurovascular unit (astrocyte-endothelium complex).18,21 It has a production rate of 0.3-0.4 ml/min (equivalent to 20 ml/h or 500 to 650 ml/day), remaining an intracerebral volume of approximately 150 ml, with an estimated turnover rate of 4 volumes/day.17,18,21
From the LV, the CSF passes to V3 through the foramina of Monro. Similarly, CSF circulation continues from V3 to V4 via the cerebral aqueduct (Sylvian aqueduct). Finally, CSF is redistributed to the subarachnoid space (cisterna magna) via the lateral (Luschka) and medial (Magendie) foramina.21–23
Absorption takes place mainly in the cranial (Pacinian) and spinal arachnoid granulations, in the leptomeningeal vessels, in the lymphatic system (cervical and abdominal by the perineural sheaths of the cranial and spinal nerves), in the space of Virchow Robin and through the olfactory nerve and nasal mucosa.17,18,24 Its maximum rate of reabsorption is 1.5 ml/min.17
Tumors of the posterior fossa
The tumor lesions of the PF are very varied, and their frequency of diagnosis has a marked pattern in relation to age and the affected anatomical structure (Figure 2). Metastases are the most frequent tumors in adults, representing approximately 30% of lesions. Around 50% of these metastases are of pulmonary origin, followed by breast, gastrointestinal, urogenital tumors and melanomas.1 Among primary tumors, meningiomas are the most common in adults.1 In children and adolescents, the frequency is determined by the location of the tumor lesion. Globally, glial tumors are the most prevalent.1 In the case of vermian lesions,1,3,25,29 medulloblastoma is common. Metastases are very rare in people under 20 years of age.1,30 Table 1 summarizes the different PF tumors according to anatomical location.
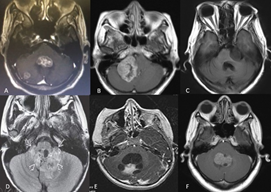
Figure 2 PF tumors. Diagnostic imaging registry courtesy Hospital Universitario del Caribe (Cartagena). (A) lung tumor metastasis in the fourth ventricle and right cerebellar hemisphere; (B) right APC meningioma; (C) left APC squamous cell tumor; (D) ependymoma; (E) right cerebellar hemisphere pilocytic astrocytoma; (F) medulloblastoma. APC, cerebellopontine angle.
Anatomical structure |
Children and adolescent |
Adults |
Cerebellar vermis Metastases |
Medulloblastoma |
Metastases |
Cerebellar hemisphere |
Gliomas (astrocytomas) |
Metastases, hemangioblastomas |
Cerebellar pontine angle (CPA) |
Rare |
1. Meningioma |
2.Vestibular schwannoma |
||
|
3. Epidermoid cyst |
|
Brainstem |
Gliomas |
Gliomas |
Fourth ventricle |
Ependymomas |
Metastases, choroid plexus papilloma |
Table 1 Most frequent tumors of the PF
Pathophysiological mechanisms and clinical repercussions
CSF is the main compensatory mechanism for intracranial pressure (ICP). This is obtained by decreasing its production and its resistance to reabsorption. Finally, there is displacement of CSF to the lumbar cistern. This happens until the increase in ICP produces a displacement of the brain structures that end up blocking the circulation of CSF, as can occur with FP tumors. Initially, the patient may be asymptomatic, or with mild symptoms (headache, gait instability). Once ICP compensatory mechanisms are exhausted, a series of clinical signs appear, such as drowsiness, impaired level of consciousness, high blood pressure and intermittent bradycardia, coma, fixed mydriasis, and even death. Consequently, intracranial hypertension (ICHT) can develop in its different stages (Table 2).16
Stage ICHT |
Intracranial response |
Clinical symptoms and signs |
1 |
CSF and CSV volume reduction – no change in ICP |
None |
2 |
Exhaustion of compensatory mechanisms – small changes in ICP |
Headache (mild symptom), drowsiness |
3 |
Rapid increase in ICP, concomitant drop in CPP |
Impaired level of consciousness (GCS), intermittent HBP and bradycardia |
4 |
Cerebral vasomotor “palsy” |
Coma, fixed mydriasis, death |
Table 2 Clinical repercussions of ICHT due to HPFt. Adapted Koenig M. Cerebral Herniation Syndromes and Intracranial Hypertension 2016. The Pathophysiology of Intracranial Hypertension and Cerebral Herniation Syndromes
CSF, cerebrospinal fluid; CSV, vascular blood content; ICP, intracranial pressure; CPP, cerebral perfusion pressure; GCS, glasgow coma scale; HBP, high blood pressure
Blockage of CSF circulation is the main cause of HPFt.12–14 In a small percentage, the pathophysiological mechanism is due to increased CSF production (eg, choroid plexus papilloma) or decreased absorption (tumors with CSF seeding and leptomeningeal infiltration, eg, ependymoma, medulloblastoma, tumor metastasis breast).29,30,32
It should be noted that, prior to performing any surgical intervention, up to 30% of pediatric patients do not present imaging signs of hydrocephalus,6–8 this percentage being higher in adults, where the absence of signs of hydrocephalus can be found up to in 90% of cases.9,10 In these patients, postsurgical hydrocephalus may develop, the pathophysiological mechanism of which is also blockage of CSF circulation due to postoperative bleeding, postoperative cerebral edema and/or secondary compression of venous outflow.1,6,12 Hydrocephalus is one of the main complications associated with surgeries for tumors located in the PF.6,12
ICHT itself does not cause brain damage, but instead produces diffuse or focal ischemia secondarily. Any large increase in ICP leads to a critical reduction in cerebral perfusion pressure (CPP). If this decrease in CPP is not controlled, it leads to global hypoxia and a general decrease in cellular activity. When ICP is around 50 to 60 mmHg, it approaches systemic arterial pressure in the circle of Willis vasculature, leading to global cerebral ischemia and ultimately brain death.33
Therapeutic approach
The main goal of all HPFt treatments is to decrease ICP.6,34,35 Within the management possibilities there are temporary measures, such as medical treatments based on hyperosmolar solutions and/or systemic steroids,17,36,37 and surgical treatments such as external ventricular drainage (EVD).1,31,32,38,39 Definitive measures refer to surgeries that seek to restore CSF circulation (endoscopic third ventriculocisternostomy -ETV- and ventriculoperitoneal/atrial shunt -VPS/VAS-) and tumor resection.29,32,40–45 There is still controversy regarding the opportune moment and the therapeutic measure of choice. Faced with this dilemma, several questions arise: 1. Is tumor resection sufficient to solve hydrocephalus? 2. When is it necessary to perform CSF diversion first? 3. Which CSF diversion treatment is the most indicated?
Next, we will try to elucidate these questions.
Medical treatment
It is useful in cases of increased ICP. There are two groups of medications that can be used: hyperosmolar solutions and systemic steroids.
Mannitol and hypertonic saline (HS) are hyperosmolar solutions that are commonly used to reduce ICP and brain volume. Several clinical trials have compared the effects of HS and mannitol on brain relaxation and lowering ICP in surgical and intensive care settings, with results indicating that HS is as effective as mannitol, and in some cases superior to manitol, this allowing a better hemodynamic stability and CPP.46–49 However, the choice of hyperosmolar solutions to be used compromises other variables, such as institutional experience, the possibility of periodic monitoring of blood pressure, renal function and electrolytes, in addition, the availability of an intensive care unit in cases where central administration is required. . Table 3 describes the characteristics and properties of mannitol and HS.
Medication |
Proposed mechanism of action |
Indication and dose |
Observations |
Manitol |
↑ plasma osmolarity, “drags” water from the cerebral intra and extracellular space to the distal convoluted tubule;17,37 it is an osmotic diuretic; ↑ FSC (improves the rheological properties of blood).47 |
Signs of ICHT and/or herniation syndrome. Recommended dose 0.25-1 g/kg of weight, in boluses every 6-8 hours. Therapeutic effect: 30-60 min, duration: 1.5-6h. |
Simultaneously administer normal saline solution 1-2 cc/kg of weight, to avoid dehydration. Monitor electrolytes and kidney function every 12 hours. |
HS |
Does not cross the intact BHE 56; causes a greater increase in serum osmolality compared with mannitol 47; has little diuretic effect 47; inhibits the expression of aquaporin 4 (AQP4) channels 57. |
Signs of ICHT and/or herniation syndrome. Recommended doses 1-2 cc/kg body weight, in boluses every 6-8 h. |
Caution in heart failure with ejection fraction < 35% Monitor serum sodium every 6 h, if > 160 mEq/L suspend administration. HS 7% requires administration via central |
Dexamethasone |
Stabilizes BBB and decreases vascular permeability (↑ angipoietin 2 (Ang2) and ↓ vascular endothelial growth factor (VEGF), respectively);50 ↑ removal of peritumoral edema;51 ↓ CSF production by inhibition of the Na+/K+ ATPase pump.31 |
Symptomatic peritumoral vasogenic edema. Mild symptoms: 4-8 mg/day, 1 or 2 doses. Moderate and severe symptoms: 8-16 mg/day, in 2 doses.53 |
Absorption at 30 minutes. Half life 36 hours. Consider non-use in asymptomatic patients. Do not administer for more than 72 hours |
Table 3 Characteristics and properties of drugs used to decrease ICP in HtFP
CBF, cerebral blood flow; BBB, blood-brain barrier; CSF, cerebrospinal fluid.
Systemic steroids improve vasogenic cerebral edema (VCE) and decrease CSF production,50,51 contributing to ICP reduction. The systemic steroids of choice is dexamethasone, due to its great anti-inflammatory potency and null mineralocorticoid activity.52,53 When the tumor lesion is accompanied by VCE, the administration of dexamethasone is indicated for 72 hours before surgery.36 However, all peritumoral VCE do not require treatment with dexamethasone.53 The anti-oedematous effects of dexamethasone depend on the dose, and this should be individualized based on the extent of the edema and the severity of the symptoms,53,54 which are classified as mild (mild headache, nausea) and moderate/severe (severe headache, vomiting, focal neurological deficit, HTEC).53 Table 3 describes the characteristics and properties of dexamethasone.
Early tumor resection
It is the treatment of choice in patients with asymptomatic hydrocephalus or with mild symptoms, both in primary tumors and in metastatic lesions.32,36,44 In patients presenting with pre-surgical hydrocephalus, tumor resection can resolve it in 70-90% in children, and up to 96% in adults.36 However, the risk of persistence of hydrocephalus, or new onset postoperatively, is estimated at 20-40% in pediatric patients1,36 and 5.7-14.3% in adults.1,9
The most important predictors of postoperative hydrocephalus (new and/or persistent) in children and adolescents are age ≤2 years, brainstem compression, moderate or severe preoperative hydrocephalus, preoperative estimation of possible medulloblastoma or ependymoma (tumors of the midline) and brain metastases.1,30 Taking the above into account, ideally, before making the surgical decision for tumor resection, the risk of postoperative hydrocephalus should be estimated. In this regard, the Canadian Preoperative Hydrocephalus Prediction Scale (CPPRH)30 is a useful tool. In this, different variables are taken into account (Table 3), with a weighted value according to its risk estimate, and whose total sum is 10 points. Patients are classified as low risk when the score is 0-4, and high risk when it is ≥ 5 (Table 4).30
Predictor |
Escore |
Age < 2 years |
3 |
Presence of papilledema |
1 |
Moderate hydrocephalus |
2 |
Brain metastases |
3 |
Preoperative tumor estimation: |
|
Medulloblastoma |
1 |
Total |
10 |
Low risk |
0-4 |
Table 4 CPPRH in children with neoplasms of the posterior fossa. Adapted Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr (2009)
In adults, the predictive factors most associated with postoperative hydrocephalus are preoperative active hydrocephalus, the petroclival location of the tumor, and vasogenic cerebral edema. The last two in relation to extraaxial tumors.55 Pilocytic astrocytomas are also associated with an increased risk of postoperative hydrocephalus in adults.1 The Frankfurt classification system to predict the postoperative requirement for CSF drainage after resection of PF tumors can be used in adult patients, with the aim of establishing the benefit of preoperative CSF drainage.55 In this classification system, tumors are divided into intraparenchymal and extra-axial, with their different variables (Table 5), and a weighted value according to their risk estimate. Patients with a score ≥3 are considered at high risk of presenting postoperative hydrocephalus, both in intraparenchymal tumors and in extra-axial. On the contrary, those with a score of 0 and 1 have low risk. The discussion is in individuals with a score of 2, who present a higher risk in cases of intraparenchymal tumors.55
Tumor category and predictor variable |
Score |
Intraparenchymal tumor |
|
Hidrocephalus |
1 |
Transependymal edema |
1 |
Surgical position |
1 |
Resection extension expectations |
1 |
Low risk: |
0-1 |
Extra-axial tumor |
|
Location: |
2 |
Perilesional edema |
1 |
Hidrocephalus |
1 |
Low risk: |
0-2 |
Table 5 Frankfurt classification system. Adapted A novel grading system for the prediction of the need for cerebrospinal fluid drainage following posterior fossa tumor surgery. J Neurosurg 132 (2020)
External ventricular drainage
It is a temporary therapeutic measure, whose main indication is the adult patient who requires emergent CSF drainage. It should be considered in adults with metastatic disease and poor clinical condition.55 It is also a good option as “prophylaxis” in adult individuals at high risk of developing postoperative hydrocephalus.32 In this sense, the Frankfur classification system can be very useful to define those with high risk (Table 5).55 It is not indicated as “prophylaxis” in the absence of clear signs of hydrocephalus.38
A duration of < 8 days is suggested with the intraventricular catheter.38 Even duration ≤4 days is related to a lower risk of complications (ventriculitis, intraparenchymal hemorrhage, intraventricular hemorrhage, and surgical site infection).55 EVD has classically been related to ascending transtentorial herniation, however, studies have not shown causality.39 In children and adolescents it is not recommended as "prophylaxis" or treatment of hydrocephalus due to tumor lesions of a primary or metastatic nature.13
There is a wide preference for ETV. In the last 20 years, much progress has been made in the development of new equipment and refinement of techniques to carry out this procedure, becoming routine in some neurosurgical care centers. In pediatric patients, ETV has shown lower complication rates than VPS/VAS.40,45 ETV is the procedure of first choice in midline tumors (medulloblastoma and ependymomas), due to its shorter surgical time and its low incidence of morbidity and mortality compared to VPS.29 In this group of tumors, it is suggested to perform "prophylactic" ETV since, when comparing it with early tumor resection as monotherapy, lower rates of postoperative hydrocephalus (of new appearance) and/or persistence of hydrocephalus have been shown in those with pre-surgical presentation. The indication of prophylactic ETV in hemispheric cystic tumors is questionable.29 ETV should be avoided in the presence of multiple macroscopic metastases. In the case of patients who have undergone ETV, they should be followed closely in the presence of CSF metastases after tumor resection surgery, due to the risk of postoperative hydrocephalus. In these situations, VPS remains a safe alternative, since it allows early specific cancer treatment without adding additional risks.31
Adults
As in pediatric patients, the trend of recent decades places ETV above VPS/VAS.41–44 ETV is the procedure of choice for the treatment of symptomatic obstructive hydrocephalus due to primary and/or metastatic tumor lesions, presenting good efficacy and a low rate of complications.32,41–44 In cerebellar metastases, the performance of ETV is similar to VPS/VAS, but at a lower cost and with less morbidity. EVT appears to be less effective in individuals with metastatic lesions with a history of neuroinfection, intraventricular hemorrhage, and leptomeningeal carcinomatosis.32 As previously described, in this group of patients hydrocephalus can be communicating, and its pathophysiology lies mainly in the decreased absorption of CSF in the arachnoid granulations (cerebral and spinal),29,31,32 therefore, it would probably be ineffective. perform a ETV. VPS/VAS is a valid alternative in patients with the aforementioned characteristics. EVT requires the presence of a subarachnoid space in the perimesencephalic cisterns, therefore, patients with brainstem compression and basal cistern collapse have an unfavorable anatomy to perform this procedure. Also in these cases, VPS/VAS is a valid alternative.32 Peritoneal metastasis secondary to VPS has been described in patients with brain metastases,58 however this remains extremely rare.32,59
It should be noted that, unlike pediatric patients, in adults there is no indication for prophylactic ETV in patients with asymptomatic hydrocephalus.13 However, the predictors of postoperative hydrocephalus (Table 5) must be taken into account when making this decisión.55
Hydrocephalus is one of the main complications associated with tumors located in the PF, and its presentation can be pre or postoperative. It is essential to estimate the risk of postoperative hydrocephalus (persistent or new onset), in order to define therapeutic measures aimed at reducing said risk. Tumor resection may be sufficient as monotherapy in adults with asymptomatic hydrocephalus, and in others with low risk of postoperative hydrocephalus (Table 4 & 5). ETV is the procedure of choice for prophylaxis and/or treatment of HPFt in pediatric and adult patients, whose estimated risk of postoperative hydrocephalus is high (Table 4 & 5). VPS is beneficial in pediatric patients with multiple metastatic lesions and/or CSF metastases. Similarly, in adults, its use is pertinent in cases of individuals with metastases who present with communicating hydrocephalus, or who present unfavorable anatomy for ETV. EVD is very useful in adults who require emergency CSF drainage, and their intraventricular catheter must be removed before four days to reduce the risk of complications.
None.
The authors declare no conflicts of interest.

©2022 Palacios, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.