Journal of
eISSN: 2373-6410


Literature Review Volume 9 Issue 5
1Undergraduate from the 4th semester Medical Course of the São Francisco University (USF), Bragança Paulista, Brazil
2Undergraduate from the 6th semester Medical Course of the São Francisco University (USF), Bragança Paulista, São Paulo, Brazil
3Department of Neurology, Pontifical Catholic University of São Paulo, Brazil
4MSc, PhD, Teacher Guidance, Brazil
Correspondence: Paulo Henrique Pires de Aguiar, Academic of the medical course of the, Pontifical Catholic University of São Paulo, Brazil
Received: October 01, 2019 | Published: October 22, 2019
Citation: Rocha MS, Avi PH, Aguiar PHPD, et al. Genetics in epilepsy, Dravet and SUDEP: A systematic review. J Neurol Stroke. 2019;9(5):290-298. DOI: 10.15406/jnsk.2019.09.00392
Introduction: Epilepsy is a neurologic condition and its patients have higher mortality rates than healthy individuals. One of the most frequent death causes in epilepsy is SUDEP. Moreover, another cause in epilepsy, although rare, is Dravet Syndrome and it’s generally diagnosed in children in their first year of life. In around 70% of the patients there is a genetic inheritance involved and several genes have been described as related to SUDEP and Dravet Syndrome which the most prevalent were DNA and belonged to the voltage-gated channel coding.
Objectives: Identify genes related do Dravet Syndrome, SUDEP and Epilepsy in literature, analyze the convergence of results, and discuss the differences that were found, contributing to the study of genetic mapping.
Methodology: Meta-analysis realized through a bibliographical research in the electronic data banks such as PUBMED, NCBI, Scholar Google and Scielo with the descriptors Epilepsy; Epilepsies, Myoclonic; Polymorphism, Single Nucleotide; MicroRNAs; Death, Sudden. The languages used to filter the articles were English, Spanish and Portuguese. The year was a filtering factor, selecting only articles published in the last 20 years.
Results: About forty (40) genes were found to be SUDEP related and the most relevant was KCNH2, appearing more frequently than other genes. Additionally, in Epilepsy without risk for SUDEP twenty-eight (28) DNA genes were found, which the most frequently mentioned was HCN2, appearing six (6) times. Among Dravet syndrome patients, only two (2) genes were described: SCN1A and SCN9A, both of them were SUDEP risk related. In epilepsy nineteen (19) microRNA genes were found in five (5) different articles.
Project: It was identified genes related to Dravet Syndrome, SUDEP and Epilepsy in literature and was noted that some genes appeared more frequently than others. Among the DNA related genes, the most prevalent were around those belonging to the voltage-gated channel coding, being them sodium, hydrogen and potassium. The results demonstrate that 25% of all articles mentioned SCN1A; 17,5% mentioned HCN2 and 15% mentioned KCNH2. Together these findings provide a basis for further investigation around the role of ionic channels in SUDEP and DS. On the other hand, regarding to MicroRNA linked genes data were scarce. Only five articles described changes on SUDEP and neither described changes compatible with DS. There was no most prevalent mRNA gene.
Conclusion: A range of studies associated DNA mutations with Epilepsy, DS and SUDEP. However, MicroRNA research could not provide concrete evidence on its influence in the development of the epilepsy and epilepsy related conditions. The identification of possible etiological factors can allow a correct and early diagnosis, providing better prognosis and therapeutic outcome, besides it can evaluate the recurrence of the disease in the family. Still, further prospective studies in larger cohorts are required to further define the genetic predisposition to SUDEP, DS and Epilepsy.
Keywords: epilepsy, epilepsies, myoclonic, polymorphism, single nucleotide, microRNAs, death, sudden
Epilepsy is one of the most prevalent neurological diseases, affecting around 50 million patients worldwide is characterized as a predisposition to the occurrence of lasting epileptic seizures and also by the neurobiological, cognitive, psychological and social consequences caused by this condition.1–3 Patients with this condition have higher mortality rates when compared to healthy individuals and in around 70% of the cases there is a genetic factor involved in the pathogenesis.2,4 Generalized Seizures, non-adhesion to treatment or drug-resistant epilepsy are the key risk factors to the occurrence of a fatal event.5
Epilepsy is a chronic neurologic condition and its etiology is diverse.6 This disease is responsible for involuntary epileptic seizures due to serious synaptic changes which increases the mortality rates in affected patients.7 One of the most frequent death causes is the sudden and unexpected death in epilepsy (SUDEP) accounting 17% of all deaths in epilepsy.8 Moreover, it has an annual incidence rate 1:1000 adults and around 0,2:1000 in children.8 Its occurrence grows as the number of epileptic seizures increases, in the presence of a night crisis, the longer the time since the beginning of the condition, early onset of the disease and cardiorespiratory comorbidities.8
In addition, another cause of death in epilepsy, despite rare, is Dravet Syndrome (DS), a spectrum of the disease characterized by the beginning of prolonged seizures in the first year of life; among those with seizures in the first year of life 3-8% have DS.9,10 In the second year of life, others types of seizures arise.9 Around 15% of the children with this syndrome dies before reaching the youth. In cases of survival, the seizures occur in monoclonal crisis, of absence and partially complex, as well as cognitive impairment, ataxia and behavioral changes.11 Unlike other types of seizures, that in many cases it is possible to manage, the same isn’t possible in patients with DS, which still does not have a treatment option.9 Dravet Syndrome is found between 1:20000 and 1:40000 people, with a prevalence two times higher in boys.10 Furthermore, those that suffer from this disease have 15 times higher risks of a SUDEP occurrence than in other pediatric epilepsies.9,7 Also, DS can be classified as a channelopathy because 80% of the patients have a mutant SCN1A gene, responsible for codifying a protein that forms the sodium transport channel between the cell membranes.11
As mentioned before the epileptic syndromes patients have a genetic inheritance involved in 70% of the cases. Because of that, the main genes were grouped together in a table relating each one to the corresponding pathologies. Scientists started to create patients’ genetic maps to research alterations, such as genome-wide single nucleotide polymorphism (SNP), to analyze and identify connected genes to Dravet, SUDEP and Epilepsy, even though the quantity of known genes is inaccurate due its large vastitude and to the dynamics of his new discoveries.
Mapping patient’s genome allows the scientific community to widen access to knowledge. Also, it contributes to precision medicine, meaning it individualizes the therapeutic management and prognosis study considering environment and genetics factors. This improves patient’s diagnosis as well as treatment efficacy and accuracy.
The main goal of this work is to identify genes related to Dravet Syndrome, SUDEP and Epilepsy in literature; to analyze convergence of results; to discuss the differences that were found and to contribute to the study of genetic mapping that allows early diagnosis and improve treatment efficacy.
In this meta-analysis study, a range of articles it was investigated through a bibliographical research in the electronic data banks: PUBMED, NCBI, Scholar Google and Scielo with the descriptors Epilepsy; Epilepsies, Myoclonic; Polymorphism, Single Nucleotide; MicroRNAs; Death, Sudden. Also, several arrangements were made among the descriptors and also a manual search along the references of the consulted articles. The languages used to filter the articles were English, Spanish and Portuguese. The year was a filtering factor, selecting only articles published in the last 20 years.
Thirty-seven (37) articles were selected and analyzed. After a more detailed examination and the build of 4 tables composed by twenty-one (21) articles, where it was realized a correlation between the gene involved and the article that cited it, we could notice that some genes appeared more frequently than others, but the mutations were in different regions.
In Dravet only two genes were found: SCN1A and SCN9A, both DNA with locus differences. All of them are related to SUDEP risks. In Epilepsy exactly nineteen (19) microRNA were found in five (5) different articles. Also in Epilepsy, without SUDEP risk, were found twenty-eight (28) DNAs and six (6) of them are different locus of HCN2, two (2) of CDKL5, two (2) of HCN1, two (2) of HCN4, two (2) of KCNH2. About SUDEP DNA genes were found forty (40), the most relevant is KNCH2, because appears in numerous studies. In resume, 21,8% of all mentioned genes are Epilepsy microRNA; 32% are Epilepsy DNA without SUDEP risk; and 45,9% are SUDEP DNA genes; 25% of all articles mentioned SCN1A; 17,5% mentioned HCN2 and 15% mentioned KCNH2.
On the next page the Table 1 exposes the main genes associated to Dravet Syndrome. In addition, the table mentions other genes disorders and its bibliographical references. SCN1A appears in three (3) different articles and locus. The gene mutations are linked to the pathologies: early infantile epileptic encephalopathy type 6 and generalized epilepsy with febrile seizures plus type 2. SCN9A is also related to Dravet Syndrome, and is associated to paroxysmal extreme pain disorder.
|
Gene |
Locus |
Sudep |
Other disorders |
Reference |
|
SCN1A |
2q24.3 |
YES |
Early Infantile Epileptic Encephalopathy type 6 and Generalized Epilepsy with Febrile Seizures Plus type 2 |
12 |
|
SCN1A |
c.4507G>A p(E1503K) |
YES |
Early Infantile Epileptic Encephalopathy type 6 and Generalized Epilepsy with Febrile Seizures Plus type 2 |
13 |
|
SCN1A |
-- |
YES |
Early Infantile Epileptic Encephalopathy type 6 and Generalized Epilepsy with Febrile Seizures Plus type 2_ |
14 |
|
SCN9A |
2q24.3 |
YES |
Paroxysmal Extreme Pain Disorder |
13 |
Table 1 Dravet syndrome
The following Table 2 presents microRNAs associated to Epilepsy. Only five (5) articles and nineteen (19) microRNA were found to be related to the disorder, and most of them couldn’t explain the specific function of each microRNA.
|
miRNA |
Function |
Reference |
|
miR-124 |
Down regulation |
16 |
|
miR-34a |
Up regulation at BCL-2 |
16 |
|
miR-128 |
Down regulation in hippocampus |
16 |
|
miR-132 |
Up regulation at CA3 in hippocampus |
16 |
|
miR-134 |
Down regulation at CREB and BDNF; Up regulation in hippocampus |
16 |
|
miR-30d-5p |
Down regulation; RBMSI - locus 2824.2; |
16 |
|
miR-92b-3p |
Down regulation; ENPP6 - locus 4q35.1; |
17 |
|
miR-130a-3p |
Up regulation |
17 |
|
miR-146a-5p |
Up regulation |
17 |
|
miR-106b-5p |
Down regulation |
17 |
|
miR-301a-3p |
Down regulation |
17 |
|
miR-let-7d-5p |
Down regulation at MIRNLET7D gene |
17 |
|
miR-23a |
Up regulation of TSC1; |
18 |
|
miR-324-5p |
Regulates cell proliferation and stem cell differentiation; targeting GUI and SMO |
16 |
|
miR-193; |
--- |
19 |
|
miR-146a; |
--- |
19 |
|
miR-30c; |
--- |
19 |
|
miR-466b; |
-- |
19 |
|
miR-301a-3p |
Down regulation; MAFB- locus 20q12; |
20 |
Table 2 microRNA in epilepsy
The upcoming Table 3 demonstrate Epilepsy risk related genes but not linked to SUDEP. Eleven (11) articles and twenty-eight (28) DNAs were found on this topic. These genes have been correlated to other disorders, most of them the different types of Early Infantile Epileptic Encephalopathy, Rigid Spine Muscular Dystrophy, Long QT Syndrome and Brugada Syndrome.
The Table 4 indicates SUDEP associated risk genes. Seven (7) articles and forty (40) DNAs were found treating this theme. These genes have been cited before in other disorders, which most were over the different types of Early Infantile Epileptic Encephalopathy, Long QT Syndrome and Cardiac Arrhythmia.
|
Gene |
Locus |
Risk of slidep |
Other disorders |
Reference |
|
MECP2 |
c.473C> T; Xq28; Mutation on p. (T158M); |
NO |
Refit Syndrome and X-Linked Syndromic Mental Retardation type 13 |
13 |
|
TSC1 |
9q34.13 |
NO |
Tuberous Sclerosis type 1 and Lymphangioleiomyomatosis |
13 |
|
CDKL5 |
c.2277-2A> C; Mosaicism; |
NO |
_ |
13 |
|
CHD2 |
c.1618G> A; Alter protein p. (V540I); |
NO |
Childhood-Onset Epileptic Encephalopathy and Lennox-Gastaut Syndrome |
13 |
|
TSC2 |
16p13.3 |
NO |
Focal Cortical Dysplasia type II and Lymphandioleiomyomatosis. |
1.3 |
|
NRXN1 |
2p16.3 |
NO |
Pitt-Hopkins-Like Syndrome type 2 |
13 |
|
CSMD3 |
8q24.11-q24.13 |
NO |
Benign Adult Familial Myoclonic Epilepsy |
13 |
|
VCL |
10q22.2 |
NO |
Dilated Cardiomyopathy 1W and Familial Hypertrophic Cardiomyopathy type 15. |
13 |
|
CDKL5 |
Xp22.13 |
NO |
Early Infantile Epileptic Encephalopathy, 2 and Rett Syndrome |
13 |
|
KCNCQ2 |
20q13.33 |
NO |
Early Infantile Epileptic Encephalopathy type 7 and Benign Familial Neonatal Seizures type 1 |
13 |
|
KCND2 |
Not confirmed |
NO |
Cycloplegia and Gastrointestinal Lymphoma |
13 |
|
SYNGAP1 |
6p21.32 |
NO |
Autosomal Dominant Non syndromic Intellectual Disability type 5 and Autosomal Recessive Mental Retardation type 5 |
13 |
|
GNA01 |
16q13 |
NO |
Early Infantile Epileptic Encephalopathy type 17; Neurodevelopmental Disorder with Involuntary Movements |
13 |
|
HCN2 |
GC+CC; rs3752158 locus C; Mutation on p.S632W e p.V246M; |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
21 |
|
HCN2 |
c.1584+7C>T |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
24 |
|
HCN2 |
E515K |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
22 |
|
HCN2 |
Insertion of 4 base pairs into nucleotide 1869 on the linker region of the gene. |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
23 |
|
HCN2 |
R527Q |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
24 |
|
HCN1 |
TT; rs10462087; |
NO |
Early Infantile Epileptic Encephalopathy type 24 and Undetermined Early-Onset Epileptic Encephalopathy |
21 |
|
HCN 1 |
A881 T |
NO |
Early Infantile Epileptic Encephalopathy |
24 |
type 24 and Undetermined Early-Onset Epileptic Encephalopathy. |
||||
|
SCN1B; |
Mutation on p.Arg214GIn- |
NO |
Early Infantile Epileptic Encephalopathy type 52 and Generalized Epilepsy with Febrile Seizures Plus type 1 |
25 |
|
CACNA1 H |
— |
NO |
Familial Hyperaldosteranism type IV and Childhood Absence Epilepsy type 6. |
26 |
|
HCN4 |
— |
NO |
Sick Sinus Syndrome type 2 and Brugada Syndrome type B |
26 |
|
HCN4 |
RSSOC |
NO |
Sick Sinus Syndrome type 2 and Brugada Syndrome type B |
27 |
|
HCN2 |
rs7255568 |
NO |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
21 |
|
KCNH2 |
c.246T>C |
NO |
Long QT Syndrome type 2 and Short Cif Syndrome type 1 |
28 |
|
KCNH2 |
p.Pro1034fs |
NO |
Long QT Syndrome type 2 and Short OT Syndrome type 1 |
29 |
|
KCNQ1 |
c.817C>T on exon 6 |
NO |
Long Qt Syndrome type 1 and Jervell And Lange-Nielsen Syndrome type 1 |
30 |
Table 3 Epilepsy risk genes.
Gene |
Locus |
Other disorders |
Reference |
SCN8A |
c.2543C> G; |
Early Infantile Epileptic Encephalopathy type 13 and Benign Familial Infantile Seizures type 5 |
13 |
DEPDC5 |
c.4427-2A> G |
Familial Focal Epilepsy with Variable Foci type 1 and Autosomal Dominant Epilepsy with Auditory Features |
13 |
SCN1 A |
c.45070> A; |
Early Infantile Epileptic Encephalopathy type 6 and Generalized Epilepsy with Febrile Seizures Plus type 2 |
13 |
SCN1A |
dbSNP A1067T; Mutation on Nav 1.1 |
Early Infantile Epileptic Encephalopathy type 6 and Generalized Epilepsy with Febrile Seizures Plus type 2 |
13 |
SCN5A |
c.1066G> A; p.(D356N), |
Cardiac Arrhythmia, Sudden Infant Death |
13 |
SCN5A |
R523C no exon 12; |
Cardiac Arrhythmia, Sudden Infant Death Syndrome and Long CI Syndrome type 3 |
31 |
KCNT1 |
c.1038C> A e 9q34.3; |
Nocturnal Frontal Lobe Epilepsy type 5 and Early Infantile Epileptic Encephalopathy type 14. |
13 |
CHRN A4 |
20q13.33 |
Nocturnal Frontal Lobe Epilepsy type I and Autosomal Dominant Nocturnal Frontal Lobe Epilepsy |
32 |
SCN2A |
2q24.3 |
Benign Familial Infantile Seizures type 3 and Early Infantile Epileptic Encephalopathy type 11 |
32 |
HCN4 |
15q24.1; |
Sick Sinus Syndrome type 2 and Brugada Syndrome type 8 |
33 |
HCN2 |
Mutations on Phe738Cys, |
Rigid Spine Muscular Dystrophy type 1 and Epilepsy |
33 |
KCNH2 |
7q36.1; Mutation Arg176Trp and Arg1047Leu; |
Long QT Syndrome type 2 and Short OT syndrome type 1 |
33 |
KCNA1 |
12p13.32; |
Episodic Ataxia type 1 and 'Isolated Autosomal Dominant 1-tvpomannesemia Glaudemans type |
32 |
KCNQ1 |
11p15.5-p15.4; |
Long at Syndrome type 1 and Jervell And Lange- Nielsen Syndrome type 1 |
32 |
COL18A1 |
21q22.3 |
Knobloch Syndrome type 1 |
32 |
JUP |
17q21.2 |
Naxos Disease and Familial Arrhythmogenic Right Ventricular Dysplasia type 12 |
32 |
SPTAN1 |
9q34.11 |
Early Infantile Epileptic Encephalopathy type 5 and Focal Epilepsy |
32 |
SCARB2 |
4q21.1 |
Epilepsia mioclonica progressiva type 4 |
32 |
CNTNAP2 |
7q35-q36 |
Sindrome da epilepsia focal por displasia cortical; Sindrome Pitt-Hopkins; |
32 |
GNAI2 |
3p21.31 |
Taquicardia ventricular id idiopatica, Adenoma hipofisario secretor de ACTH; |
32 |
KCTD7 |
7q11.21 |
Myoclonic Progressive Epilepsy type 4 and Unvercicht-Lundborg Syndrome |
32 |
DPP6 |
7q36.2 |
Autosomal Dominant Mental Retardation type 3 and Paroxysmal Familial Ventricular Fibrillation type 2 |
32 |
PCDH19 |
Xq22.1 |
Childhood Absence Epilepsy and Early Infantile Epileptic Encephalopathy type 9 |
34 |
RYR2 |
nsSNP Q2958R |
Catecholaminergic Polymorphic Ventricular Tachycardia type 1, with or without Atrial Dysfunction and/or Dilated Cardiomyopathy and Familial Arrhythmogenic Right Ventricular Dysplasia type 2 |
35 |
RYR2 |
GABRG3 Duplication |
Catecholaminergic Polymorphic Ventricular Tachycardia type 1, with or without Anal Dysfunction and/or Dilated Cardiomyopathy and Familial Arrhythmogenic Right Ventricular Dysplasia type 2 |
36 |
RYR3 |
nsSNP C1288Y |
Familial Arrhythmogenic Right Ventricular Dysplasia type 2 and Central Core Myopathy |
35 |
5-HT |
nsSNP R260H |
_ |
35 |
CACNA1 A |
p.R2241W; |
_ |
34 |
CACNA1C |
p.P2421V; 19p13 |
Timothy Syndrome and Brugada Syndrome type 3 |
34 |
EFHC1 |
_ |
Myoclonic Juvenile Epilepsy and Juvenile Absence Epilepsy type 1. |
34 |
SCN11A |
p.C844Y |
Familial Episodic Pain Syndrome type 3 and Hereditary Sensory and Autonomic Neuropathy type VII |
34 |
SCN10A |
p.M1267R |
Familial Episodic Pain Syndrome type 2 and Sodium Channelopathy-Related Small Fiber Neuropathy |
34 |
FBN 1 |
_ |
Stiff Skin Syndrome and Marfanoid-Progeroid- Lipodystrophy Syndrome |
34 |
SCN4A |
p.R1828C |
Paramyotonia Congenita Of Von Eulenburg and Potassium-Aggravated Myotonia |
34 |
ARRB2 |
_ |
Whim Syndrome and Bardet-Biedl Syndrome type 19 |
36 |
ANK2 |
4q25-q26 |
Arrhythmia, Ankyrin-B-Related Cardiac Arrhythmia and Long Qt Syndrome type 1 |
36 |
COL18A1 |
21q22.3 |
Knobloch Syndrome type 1 |
36 |
CNTNAF2 |
7q35-q36 |
Pitt-Hopkins-Like Syndrome type 1 and Autism type 15 |
36 |
CASK |
Xp11.4 |
Mental Retardation and Microcephaly with Pontine and Cerebellar Hypoplasia, FG Syndrome type 4 |
36 |
Table 4 Sudep risk genes
Genes related to Dravet Syndrome, SUDEP and Epilepsy have been identified in literature and was noted that some of them appeared more frequently than others. The results demonstrate that 25% of all articles mentioned SCN1A; 17,5% mentioned HCN2 and 15% mentioned KCNH2.
Among the DNA related genes, the most prevalent were around those belonging to the voltage-gated channel coding, being them sodium, hydrogen and potassium. The Sodium voltage-gated channels (SCN) appeared more frequently, being cited fourteen times which four of them were SCN1A. Also, mutations in SCN1B, SCN2A, SCN5A, SCN8A, SCN9A, SCN10A, SCN11A, despite less recurrent were also described. The Hydrogen voltage-gated channels (HCN) were mentioned in twelve occasions of which seven pointed to HCN2. Besides, variations were also seen in HCN1 and HCN4. Finally, the Potassium voltage-gated channels were reported in nine occasions of which three were about KCNH2. Moreover, mutations have been cited in KCNH1, KCNQ1, KCNT1, KCNQ2, KCNA1, KCND2. Together these findings provide a basis for further investigation around the role of ionic channels in SUDEP and DS.
SCN1A
It is related to DS, also denominated as early childhood epileptic encephalopathy type 6.11–13 In this disease, particularly, there are 12 possibilities of deleterious alterations observed, being 50% missense type (c.829T> C, c.971A> C, c.2360T> G, c.4093G> T, c.5178G> T and c .5434T> C), 25% splice sites (IVS2 + 1A> G, IVS4 + 1G> A and IVS8 + 3G> T), 17% frameshift type (c.3719_3720insGATA and c.1242delA), 8% deletion of a triplet (c.5489_5491delAGT).14 Mutations provide a great risk of SUDEP, because this is a rare and severe progressive encephalopathy without concrete treatment.13,14,29,37
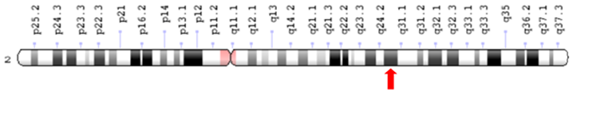
Image 1 Cytogenetic location of SCNA1 gene: 2q24.3. Adapted from: Genetics Home Reference.38
Modifications in SCN1A gene are also linked to generalized epilepsy associated with febrile seizures type 2.14,37 According to article research, the proteins responsible for the composition of Nav1.1 (voltage-dependent sodium channel type I, alpha subunit) are modified, also altering the synaptic functions of cells.13,14
KCNH2
With the intention of connecting canalopathic epilepsy with cardiac arrhythmias, the KCNH2 gene was experimented.29 Observed modifications in 7q36.1, which changes Arg176Trp and Arg1047Leu13; also p.Pro1034fs (c.3101_3108del), in this case there is a premature stop codon that gives the degradation order of mutated mRNA; the missense heterozygous mutation (c.246T> C) in the second exon of the gene leads in the alteration of isoleucine (p.I82T).28,29,33 This is a gene responsible for the composition of Kv1.1, potassium channels, and its alterations are linked to type 2 long and short QT syndromes, as well the epilepsies.28,29,33

Image 2 Cytogenetic location of KCNH2 gene: 7q36.1. Adapted from: Genetics Home Reference.39
Theories suggest the concept of cardiocerebral channelopathy, because they are the sites of highest expression of proteins encoded by the KCNH2 gene.28 The hERG channels, encoded by KCNH2, can regulate neuronal triggering and are particularly active in astrocytes: disturbances of this channel may confer susceptibility to recurrent seizure activity.28,40–42
HCN2
It is the gene responsible for coding the H channel, subunit number 2, for activated cations by cyclic nucleotide and for hyperpolarization,21–23 has high sensitivity to cAMP modulation, and is located especially in the thalamus and trunk region. Studies have identified modifications in HCN2 Gs rs7255568 and rs3752158 alleles in genetically generalized epilepsies (GGEs) determined, regarding to controls (p=0.039, p=0.027, respectively).21 Only rs7255568 was linked to risks of childhood absence epilepsy (CAE) (p=0.028) and juvenile myoclonic epilepsy (JME) (p=0.02).21 While rs3752158 is linked to the risk of generalized tonic-clonic seizures, JME and febrile seizures, in all C and GC + CC alleles.21,33 Changes in the two previously mentioned alleles, as well as variations of R527Q in HCN2 exon 5 are also present in congenital rigid spine muscular dystrophy.21,33 There are few studies that address R527Q and these are not conclusive when its importance in GGEs.21,33 Regarding idiopathic generalized epilepsy (IGE) in the HCN2 gene, numerous variants were found, the most prevalent was the substitution of amino acids R527Q in exon 5.21,24
The intron 5 variant (c.1584 + 7CNT) was related to epilepsy from family history studies.21 Such research leads us to believe that changes in channels with this variant cause a decrease in conductance-voltage.24 It was identified a type of missense mutation in exon 5 of the HCN2 gene, which causes mutation of the E515K protein, which may be related to sporadic generalized epilepsy. Such mutation involves the Glu515 residue, which is of great importance for channel functionality, as it is involved in triggering. Alterations imply loss of channel function, increasing its excitability at rest.22 It was identified 4 base pair insertion, TTCA, in nucleotide 1669 in exon 6 of the HCN2 gene at the ion channel.23 This mutation compared when naturally occurred and when induced provide base shifting and the addition of 41 amino acids and a different stop codon.23 Producing, therefore, absence epilepsy and ataxia, both of early manifestation.23
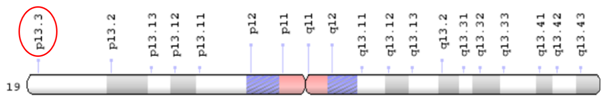
Image 3 Cytogenetic location of HCN2 gene: 19p13.3. Adapted from: Genetics Home Reference.40
On the other hand, regarding to MicroRNA linked genes data were scarce. Only five articles described changes on SUDEP and neither described changes compatible with DS. The changes cited covered the genes: miR-124, miR-34a, miR-128, miR-132, miR-134, miR-30d-5p, miR-92b-3p, miR-130a-3p, miR-146a-5p, miR-301a-3p, miR-let-7d-5p, miR-23a, miR-324-5p, miR-193, miR-146a, miR-30c, miR-466b. There was no most prevalent gene.16–20
In this meta-analysis, the tables made it possible to assemble the main genetic components that, mutated, may express different conditions (Tables 1-4). We report a range of studies associating DNA alterations with epilepsy, DS and SUDEP (Tables 1,3 & 4). This series of highly scrutinized DNA analyses will allow advances in early diagnosis.23,33 For DS, only two genes were found, SCN1A and SCN9A, both related to the risk of SUDEP (Table 1). For epilepsy with or without risk of SUDEP were found mainly SCN1A, HCN2, KCH2 (Table 3). However, research involved in mRNA didn’t provide concrete evidence on the influence on different diseases investigated, only suggested the possibility of interference (Table 2).
The combination of genetic effects with susceptibility factors for developing other mutations contributes to the increased risk of SUDEP (Table 1). The pathological relation between brain and heart in SUDEP is evident, because the review demonstrates that K+ and Na+ channels suffer strong genetic interference, and, such channelopathies lead to high mortality when there is no adequate treatment.13,28,31,32,37 The correlation of SUDEP with other syndrome allows an accurate diagnosis search.23,28,33
Genetic tests to prove the disease are highly recommended for patients with DS and epilepsy.9,13,17 The identification of etiological factors in their early stages and the fast and appropriate procedure allow the correct and early diagnosis, providing better prognosis and therapeutic outcome; more so, it can evaluate the recurrence of the disease in the family. It can be observed, in some cases, a pattern in the disturb from the discovery of genotype.11,13,14
The mapping of a patient’s genome makes it possible to construct of a database for genetic mutations in Epilepsy, SUDEP and Dravet. It also allows the interpretation of disease etiology. It contributes to widen access to knowledge for the scientific community and to precision medicine. That’s important not only to provide options and recommend the better therapeutic management based on the real genetic cause of the disease, but also to make patient’s prognosis clear.40,43
All patient care in this process should always aim the best prognosis, even when there are serious phenotypes, which is because such conditions have consequences that are not only physiological, but also cognitive, psychologic and social, directly affecting the quality of life of the pacient.11,13,14,34 Further studies in larger cohorts are required to define the genetic predisposition.13
None.
The authors declare that there are no conflict of interest.
None.

©2019 Rocha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.