Journal of
eISSN: 2373-6410


Research Article Volume 12 Issue 3
1Neurólogo pediatra, Fundación Clínica Infantil Club Noel, Cali, Colombia
2Sección de Neurología Pediátrica, Hospital Universitario Vall d’Hebron, Barcelona, Spain
3Neuropsicóloga, Hospital Sant Joan de Déu, Barcelona, Spain
Correspondence: Eduardo Fonseca Santos, Neurólogo pediatra, Fundación Clínica Infantil Club Noel, Cali, Colombia
Received: March 05, 2022 | Published: June 14, 2022
Citation: Fonseca-Santos E, Macaya A, Aloy LM. Evaluation of Visual Cognitive Dysfunction (VCD) in patients with a history of neonatal hypoglycemia. J Neurol Stroke. 2022;12(3):61-67. DOI: 10.15406/jnsk.2022.12.00502
Glucose is essentially important for brain metabolism, it has a dual function for normal brain development: as an important metabolic fuel, and as a precursor of essential macromolecules during the rapid growth phase of the brain.
It is well known of the severe outcomes of hypoglycemia in the neonatal period predominantly affecting the occipital lobes.1–5 The neurological sequelae of severe or symptomatic hypoglycemia are neonatal encephalopathy including delayed psychomotor development, learning or behavioral problems, epilepsy and visual impairment.6 The severity of this type of injury depends on the levels and duration of hypoglycemia and the existence of other comorbidities.
Visual problems reported in studies of neonates with hypoglycemia are highly prevalent and mostly limited to reporting visual defects such as strabismus (esotropia/exotropia), optic atrophy and cortical visual field defects.7 Also reports of visual loss with abnormal visual evoked potentials would be the most frequent visual disturbances documented.
Despite the prevalence of neonatal hypoglycemic encephalopathy, studies on Cortical Visual Impairment (CVI) are scarce, debatable and the consequences for long-term visual outcome are also unclear.
Relatively little research has been done to quantify visual skills in these children who often cannot describe what they can see (nonverbal response). Therefore, one of the ways to measure visual-spatial skills would be by means of visual perception tests, which can be quantitative and do not require verbal responses.
To evaluate Cortical Visual Disabilities (CVD): defined as a neurological disorder caused by lesion or malfunction of retro-geniculate visual pathways (optic radiations, occipital cortex, visual associative areas) in the absence of significant ocular disease in a group of patients with a history of hypoglycemia through different visual tests: Visual evoked potentials (VEP) and campimetry and also indicate the characteristics of the lesion in brain magnetic resonance imaging (MRI) and electroencephalogram (EEG). To describe possible Visual Cognitive Dysfunctions (VCD) in the study patients: defined as alterations in the ability to analyze and process visual information, through visual perception and memory tests, and manifested as a disorder of visual-perceptual skills; and to compare their results with a control group.8
Describe and discuss possible correlations of visual perception and memory test results with neurophysiological test findings.
This is a descriptive observational study of a historical cohort of children with a history of neonatal hypoglycemia who underwent prospective evaluation with sample size by convenience; the tests are part of the routine evaluation by clinical practice of the follow-up of patients with perinatal pathology (who presented neonatal hypoglycemia). The study was conducted in the period from May to September 2014 at the Vall d'Hebron University Hospital, Barcelona in Spain.
Inclusion criteria
Patients between 8 and 18 years of age (age range of application of the selected neuropsychological tests), with a history of neonatal hypoglycemia. Cases were identified through the review of medical records prior to 2005, including all neonates with symptomatic or asymptomatic neonatal hypoglycemia but with blood glucose levels < 45 mg/dl requiring intravenous glucose infusions.
Exclusion criteria
Extremely preterm newborns < 30 weeks (due to the risk of perinatal brain injury from other causes) or neonates with brain pathology secondary to perinatal hypoxic-ischemic encephalopathy (defined as a combination of neonatal encephalopathy, umbilical cord arterial pH of less than 7.0, Apgar score less than or equal to 5 at one minute and five minutes). Patients with a history of neonatal hypoglycemia, but with neurological sequelae (severe mental retardation or cerebral palsy) that prevent or make it impossible to perform the tests. Patients with pathology of the anterior visual pathway or ocular structures.
The control group is a population matched by age and sex with the case group, coming from a historical cohort evaluated by the Neuropsychology service and coming from voluntary participants with no known relatives of the case-study patients and with no pathological history in the neonatal period.
For the classification of neonatal hypoglycemia, when reviewing the scientific literature, there has not yet been a consensus among experts on the cut-off value to define neonatal hypoglycemia, but we opted for the concept of any newborn with signs and symptoms compatible with hypoglycemia and glycemia below the most widely accepted lower limit: <2.6 mmol/l (47 mg/dl).9,10
We classify neonatal hypoglycemia in 4 categories: 1) "transitional-adaptive" hypoglycemia, generally transient, manifested in the immediate postpartum period, responds immediately to glucose administration, is frequently observed in neonates of diabetic mothers or newborns with fetal erythroblastocytes. 2) Secondary hypoglycemia - related, early onset, mild and with rapid response to glucose administration. It is associated with hypoxia, intracerebral hemorrhage, sepsis, etc. 3) Classic-transient hypoglycemia occurs towards the end of the first day, of moderate to severe degree. It usually requires prolonged treatment. It is seen in low birth weight newborns with depletion of glycogen and lipid stores. 4) Recurrent hypoglycemia variable onset over time, neonates with severe and prolonged hypoglycemia. It is observed in neonates with pancreatic β-cell hyperplasia and inborn errors of metabolism.11
To identify risk factors associated with neonatal hypoglycemia and brain injury, medical records were reviewed for possible associated factors such as maternal condition (maternal diabetes mellitus and pre-eclampsia); acute fetal distress, delivery (vaginal or cesarean section), sex, newborns were classified as term with gestational age greater than or equal to 37 weeks and small or large for gestational age when their weight was less than the 10th percentile or greater than the 90th respectively; Apgar score at 1 and 5 minutes, the degree of hypoglycemia level and the duration of hypoglycemia. Neonatal seizures were diagnosed with clinical symptoms and were defined as paroxysmal alteration in neurological function, i.e., motor behavior, and/or autonomic function, in the neonatal period.12
In the imaging study, Siemens Magnetom Avanto-1.5T magnetic resonance imaging was used, performing T1-, T2- and FLAIR-weighted sequences in the 3 spatial planes. The images were evaluated by the neuroimaging service of the Vall d'Hebron University Hospital (experienced neuroradiologists). Lesion findings were analyzed to establish possible relationship with the primary optic pathway, parieto-occipital regions (dorsal pathway), or temporal and infero-temporal regions (ventral pathway).
The neuropsychological examination began with:
In the test battery for visual object and spatial perception: VOSP, they were initially given the shape detection test (an adaptation of Warrington and Taylor's figure/background perception test) and if the results of this test showed significant deficits and did not exceed 15 points, the individual was considered unsuitable to continue with the VOSP test (Table 1 & 2).
Test |
Acronym |
Evaluates |
Number of items |
Description |
Judgment of Line Orientation |
JLO |
Assesses the ability to perceive the |
5 test |
The subject is asked to classify |
Hopper's visual organization |
HVOT |
Assesses the ability to organize visual |
30 sheets |
It consists of 30 drawings of |
Visual object and spatial perception |
VOSP |
1-4 substest assesses visuoperceptive |
9 subtests |
It consists of nine tests: shape |
Table 1 Visual Perception Tests
Test |
Acronym |
Evaluates |
Number of items |
Complex figure of King |
FCR |
Assesses visuoconstructive ability and |
1 complex figure |
Table 2 Visual Memory Tests
Contingency tables (to compare qualitative variables)
Analysis of covariance (to eliminate the effect of other variables that influenced the performance).
A comparison of the BAS-II School cognitive tests, visual perception, visual memory and CPT-II continuous performance between the case group (history of hypoglycemia) and the control group was carried out; for these group comparisons it was taken into account that in the age percentiles, the two groups presented equal percentage of age intervals and gender ratio. Performances less than or equal to the 13th percentile (a t equal to or less than 39) is considered lower-scoring evidence. Intergroup comparison with P < 0.001 is considered statistically significant.
Sixteen patients (13 men and 3 women) met the criteria for this study, of whom 11 patients (10 men and 1 woman) agreed to participate in the study. Three were unable to participate because they were out of the country and in two the parents did not authorize their participation for personal reasons.
The selected children ranged in age from 9 years to 13 years. The mean age at examination was 11.6 years; their mean gestational age at birth was 38 weeks (range 30-42 weeks). The time of onset of hypoglycemia was variable, and could persist despite early glucose treatment. In our study a high percentage manifested as recurrent hypoglycemia in 72.7% (8/11), followed by classic hypoglycemia- transient 18.1% (2/11) and secondary hypoglycemia- related 9% (1/11) (Table 3).
# Patient |
G/V/S |
Apgar 1'. |
Apgar 5'. |
Weight/P-th |
N blood glucose |
Duration |
Ranking |
Clinic |
1 |
39/d/m |
9 |
10 |
2800 (10th). |
<30 mg/dl |
8 hr |
HR (ABCC8) |
C,S |
2 |
39/d/m |
9 |
10 |
3470 ( 50-75th) |
<30 mg/dl |
1 hr |
HR |
S |
3 |
37/d/f |
9 |
10 |
3310 ( 75-90 th) |
12 mg/dl |
8 hr |
HR (ABCC8) |
C,S |
4 |
38/d/m |
9 |
10 |
3360( 75-90 th) |
42mg/dl |
12 hr |
HR (ABCC8) |
C,S |
5 |
40/c/m |
5 |
9 |
3700 ( 75-90th) |
<45mg/dl |
* |
HR |
S |
6 |
38/c/m |
3 |
7 |
2290 ( <5 th) |
<45mg/dl |
1 hr |
CT |
S |
7 |
30/c/m |
* |
* |
1680 ( 75-90 th) |
<45mg/dl |
* |
HR |
A |
8 |
37/c/m |
9 |
10 |
3780( 97 th) |
20 mg/dl |
1 hr |
HR (ABCC8) |
S |
9 |
39/d/m |
9 |
10 |
2420 ( <5 th) |
<45 mg/dl |
* |
HR |
A |
10 |
42/d/m |
9 |
10 |
2570 (5-10 th) |
18 mg/dl |
1 hr |
CT |
C,S |
11 |
39/d/m |
8 |
9 |
3040 ( 5-10th) |
10 mg/dl |
1 hr |
HS |
S |
Table 3
G: gestation weeks; V, vay of delivery; d, delivery; c, cesarean section; Sex, male (m) or female (f).
*Not found. ABBC8 gene.
Classification: TA, transitional adaptive; HS, secondary hypoglycemia; CT, classic transient; HR, recurrent hypoglycemia
Classification: TA, transitional adaptive; HS, secondary hypoglycemia; CT, classic transient; HR, recurrent hypoglycemia
A high percentage of our patients (90.9%) had an adequate Apgar at birth, at 1 minute ≥5/10 and at 5 minutes ≥7/10, so asphyxia and hypoxia would not be associated risk factors, only one patient (#6) had a low Apgar (3/10) at 1 minute due to breech presentation that required forceps, but his Apgar at 5 minutes was adequate (7/10) and the umbilical cord sample reported a PH art: 7.05 - ven: 7.25; as a risk factor we would have her low weight for her gestational age (2290 gr/38 weeks) which corresponds to IGR (< P 5 th). Patient #7 with maternal history of suspected chorioamnionitis at 30 weeks of gestation, required cesarean section, newborn with a birth weight of 1680 grams (P 75-90 th), no Apgar record was found, but an arterial PH of 7.33 was documented and recurrent hypoglycemia was documented due to hyperinsulinism. As for maternal risk factors, gestational diabetes (patient #5) and preeclampsia (patient #9) were recorded. The route of delivery was generally vaginal (7/11). As for gender, 90.9% (10/11) were male. The manifestations and forms of presentation of neonatal hypoglycemia were generally symptomatic 72.7% (8/11), of which 36% (4/11) presented with seizures and only 18% were asymptomatic (2/11) (Table 3).
Ten patients in the study had EEG recorded in their neonatal period, where possible background abnormalities were reviewed, and in case of epileptiform discharges, their location, morphology, and topography were defined. Of these only two patients presented altered EEG, patient #10 who manifested seizure reported generalized epileptiform abnormalities, the other patient #7 presented focal paroxysmal alterations in the left hemisphere, but in this patient no seizures were ever documented in his neonatal period (Table 4).
# Patient |
EEG |
MRI |
VEP flash |
VEP pattern |
VF RE right eye |
VF RE left eye |
Comorbidity |
|
1 |
N |
A |
- |
A |
D |
D |
E |
|
2 |
N |
A^ |
N |
A |
- |
- |
MDH and C |
|
3 |
- |
A* |
N |
N |
- |
- |
Nc |
|
4 |
N |
N |
N |
N |
N |
N |
Nc |
|
5 |
N |
N |
N |
N |
Nf |
N |
MDH and C |
|
6 |
N |
A |
N |
N |
Nf |
D |
MDH and ADHD |
|
7 |
A |
N |
N |
N |
Nf |
Nr |
ADHD |
|
8 |
N |
N |
N |
N |
D |
D |
Nc |
|
9 |
N |
N |
N |
A |
Nf |
Nr |
Nc |
|
10 |
A |
A |
- |
N |
Nf |
Nr |
E |
|
11 |
N |
A |
N |
N D |
Nr |
ADHD |
||
Table 4
EEG, electroencephalogram; MRI, magnetic resonace imaging; VEP, visual evoked potentials; VF, visual field; RE, right eye; LE, left eye; N, normal; A, altered; -, not performed; A^, hypogenesis of corpus callosum;
A*, bilateral frontal polymicrogyria, Nr: not reliable; D, decreased or decreased sensitivity.
Comorbidity, MDH maturational delay/hypotonia; E, epilepsy; S, seizure; ADHD, attention deficit hyperactivity disorder; Nc, no comorbidity.
Neuroradiological findings on MRI: Patient #1 presents cortical atrophy-occipital lobes. Patient #2 with findings of hypogenesis of the corpus callosum without lesions due to hypoglycemia and of note also course with altered VEP pattern. Patient #3 with findings of bilateral frontal asymmetry with dysmorphic appearance of the zonal gyri in relation to probable bilateral frontal polymicrogyria. No evidence of occipital gliosis suggestive of perinatal hypoglycemia injury. Patient #6 with findings of subtle bilateral posterior occipital subcortical subcortical white matter lesion, topography at the level of the optic radiations. Patient #10 presence of leukoencephalopathic lesions in both parietooccipital regions. Patient #11 chronic gliotic lesions affecting peri-ventricular and deep white matter, standing out at the level of both the frontal lobes, centroemiovial regions as well as periartrial and periventricular regions of left predominance (Table 4 & Figure 1).
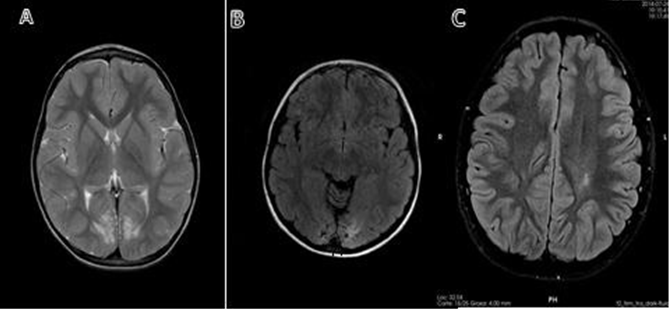
Figure 1
A) Patient #1. MRI in T2, axial slice, bilateral occipital atrophy with white matter involvement.
B) Patient #10. MRI in FLAIR, axial cut, occipital atrophy on both sides.
C) Patient #11 MRI in FLAIR, axial section, chronic gliotic lesions affecting periventricular and deep white matter, standing out at the level of central semi-oval regions, predominantly left.
In the psychomotor development and neurological findings explored periodically, seven patients in the study presented comorbidities that manifested mainly as maturational delay with hypotonia in their infant-school period; and attention deficit hyperactivity disorder (ADHD). Neuropsychological assessment results (NPA) (Figures 2-5).
In the different tests performed in the two groups (intergroup comparison) we found several differences, some of which were statistically significant (P<0.001), such as in the BAS-II test: differences in the standard cubes, alternative cubes and figure recall tests; as well as in the JLO test and discrimination and copying in the FCR test. On the other hand, other tests such as immediate and delayed memory and recognition of the FCR test were not statistically significant.
Intergroup comparison
In the BAS-II group field index, we did not find statistically significant differences in the verbal index and non-verbal rationales, but we did find significant differences in the visuospatial evaluation (P<0.001), in which the control group benefited (better results), so the overall index in the case group is low. In the CPT-II test, the behavior of both groups was very similar, so we did not find alterations in the attention capacity.
In the test battery for visual perception of objects and space: VOSP, we found statistically significant differences (P<0.001) in visual perception, spatial localization, selective attention, visual tracking and mental rotation. In terms of intra-group results, when performing the possible correlations between the different clinical, neurophysiological and imaging variables, we found that in terms of the form of presentation of neonatal hypoglycemia, the recurrent form predominated, but there were no statistically significant differences with the other forms of presentation of neonatal hypoglycemia.
Likewise, the symptomatic manifestations of hypoglycemia predominated over the asymptomatic ones, there are no statistically significant differences, this because it is a small sample of patients, but this small but appreciable percentage of 18% of asymptomatic is a fact to take into account in the evaluations and reviews of the newborn, as shown in the study where the 2 patients (# 7 and 9) of recurrent hypoglycemia caused by hyperinsulinism were asymptomatic, but with risk factors (one 30 weeks premature and the other with low weight for gestational age respectively), for which immediate and progressive adjustments of glucose infusions were implemented, in summary, analytical determinations of protocol and routine in the neonatal care services to avoid the development of complications and sequelae due to hypoglycemia.
In the neuroimaging correlation of the central nervous system (CNS) (unaltered vs altered MRI), we found anappreciable statistical difference (p < 0.004), in the JLO test as well as in the correlation of the VEP-Pattern (Normal vs Altered) but being statistically significant (p < 0.001) in the JLO test.
The most common cause of recurrent and persistent hypoglycemia in the neonatal period is persistent hyperinsulinemic hypoglycemia (PHH).13 PHH is characterized by inappropriate and unregulated insulin secretion from pancreatic β-cells in the presence of low blood glucose concentration. Early recognition, diagnosis, and prompt treatment are important, as delay in diagnosis can lead to brain injury from hypoglycemia. PHH is a heterogeneous condition in terms of clinical presentation, histology and molecular genetics. Among the most common causes of congenital hyperinsulinism are mutations in the ABCC8 and KCNJ11 genes that cause the most severe forms of PHH, which is often medically unresponsive.14 Eight of our patients were caused by recurrent hypoglycemia, of which four had mutation of the ABCC8 gene and of these three manifested with seizures, so there would be a possible correlation with its evolution and its paroxysmal manifestation by not responding favorably to the established guidelines (high infusions of glucose >8 mg/kg/min and diazoxide).
Of the 6 patients with altered MRI, only 4 corresponded to findings of hypoglycemia injury, of which 3 (# 1, 6,10) had mainly involvement of the posterior white matter (occipital lobes) as shown by most studies of neonates with acute neurological dysfunction due to hypoglycemia, but also a third of patients may present a wider distribution of lesions as would be our patient # 11.15 In addition to these alterations in the MRI, only one patient (#1) had an altered VEP Pattern, the other patients had 3no alterations in the VEPs. Two patients (#2 and 3) had morphological alterations in their MRI (one with hypogenesis of the corpus callosum and the other with probable frontal polymicrogyria) but these are not secondary findings or events due to neonatal hypoglycemia.
Patient #2 with corpus callosum hypogenesis, and known contribution of the corpus callosum in visual functions,16 in addition to his history of neonatal hypoglycemia, his neurological defect could aggravate his visual impairment, as has been documented in comparative studies of inter- and intra-hemispheric somatosensory functions in children with partial and complete agenesis of the corpus callosum.17
In the study, when performing visual fields, the vast majority of patients presented unreliable results upon interpretation, and a high percentage had no previous studies (2-3 consecutive fields), making it impossible to give correlations or interpretations. In our study, patients with a history of neonatal hypoglycemia presented a low percentage (18%- 2/11) of symptomatic occipital lobe epileptic syndrome during their evolution and follow-up (patients #1y10), these findings differ from other published reports, these findings, unlike other published reports, show high percentages up to 50%, most of them being of occipital focal origin (posterior pattern),18,19 but generally in these publications hypoglycemia is associated with asphyxia and hypoxia that would increase the risk of seizures, contrary to our study this risk factor was excluded. Another study excluded any history of asphyxia, but in the group of patients, the blood glucose value had been recorded at 20 mg/dl or lower20 where refractory seizures were observed in 42% of patients with a history of neonatal hypoglycemia.
In the interictal EEG of patient #10 who presented altered EEG, we could have a possible clinical correlation (seizure), EEG (generalized paroxysmal activity) and pathological MRI image (leukoencephalopathic lesions in both parieto-occipital regions). According to this correlation neonatal hypoglycemia can be considered as an etiological factor of symptomatic occipital lobe epilepsy in infancy.21,22
In summary, we could suggest that before these findings of a history of neonatal hypoglycemia, epilepsy, MRI alterations and visuoperceptive difficulties, they manifest as a clinical spectrum or neurological syndrome. The role of VEPs in confirming the diagnosis of CVI (Cortical Visual Impairment) in children and predicting visual outcome has been addressed in many studies, and is not free of controversy. Different technical methods, such as "Flash" and "Pattern", are used to record the potentials, that is how Clarke and collaborators23 found a low positive predictive value (45.1%) for flash VEP, other authors have shown that a poor VEP result does not necessarily mean a poor visual outcome in a present future. VEPs seem to be a useful complementary tool, but they have limitations, and we should not rush to predict a poor outcome solely on the basis of these findings,24,25 but they should be followed up as changes or improvements depending on the maturation of the sensory pathway.
VEP (Flash-Pattern) performed on the patients, we found that 10 patients who underwent flash VEP had normal results and only three patients had altered VEP Pattern (Patient # 1, 2 and 9), but with the singularity that only patient #1 had lesions due to neonatal hypoglycemia in his MRI, the others did not have pathological MRI due to neonatal hypoglycemia, for its interpretation we would have to take into account that the VEP are functional tests, while MRI is a morphological finding. A possible explanation and correlation for these unexpected findings in the VEP pattern in these 2 patients (#2 and 9) would be the possibility of performing later studies by positron emission tomography; in which it has been documented that the absence of ischemic lesions in the occipital lobe have shown that the metabolic abnormalities are larger than the corresponding anatomical abnormality shown in the MRI, and that the areas distant from the ischemic lesion may have decreased metabolism. Also thus, it has been documented that the mere presence of a normal occipital VEP flash cannot be taken as evidence of absence of sufficient cortical damage to produce loss of visual function. Therefore the presence of normal flash VEP does not necessarily suggest a favorable prognosis.26
In the results of NPA, we should keep in mind that in the perinatal period generally the first acquired lesions of the visual system are usually associated with extensive involvement of the retrochiasmatic visual pathways, leading to a wide range of visual dysfunctions, while selective lesions of the striate cerebral cortex are rare, but the adaptive plasticity of the brain in newborns is an important factor for visual recovery,27,28 and generally from 6-10 years of age,29 there will be a natural development of visual cognitive functions where assessment and exploration of visuospatial perception will be ideal.
In our case group study, their results document a selective impairment of higher visual functions, affecting visual object recognition and visuospatial skills. These findings are very similar to a previous study (case report) in a patient with recovery of visual functions after early acquired occipital injury.30
For our study, we chose the term VCD which reflect an impaired ability to process visual information. A generalized involvement of the higher visual system processing is the involvement of both ventral and dorsal pathways (the ventral pathway extending from the striate cortex to the infero-temporal cortex plays an important role in object recognition, the dorsal pathway extending from primary visual cortex V1 to the parietal-occipital cortex is involved in visuospatial skills and in control of visuo-motor actions). We note in our study that lack of more significant alterations in brain MRI does not exclude the possibility of further malfunction of the visual system processing, expressed through discrete VCD. The absence of structural lesions does not guarantee intact circuits.
The other term is CVI, defined as a neurological disorder caused by damage or malfunction of retrogeniculate visual pathways (optic radiations, occipital cortex, associative visual areas) in the absence of significant ocular disease.
Studies have mainly focused on neuro-ophthalmological aspects, such as visual acuity and visual field deficits, and common ocular motor dysfunction. However, in his 2001 revision of the definition of CVI, Good proposed to broaden the clinical spectrum of impairment to include VCD which, being related to alterations in the ability to analyze and process visual information, manifest through a higher impairment of visual skills. These dysfunctions, also grouped under the heading "visual perceptual impairment", may be associated with the neuro-ophthalmological dysfunctions typical of VCD, or constitute the main clinical manifestations of VCD in patients with normal or near-normal visual acuity.31
The use of an extensive battery of tests designed to analyze visual integration and visual-perceptual skills, demonstrated in our patients with a history of neonatal hypoglycemia a disorder for the analysis of information and visual processing, with the particularity that this impairment is not related to an attention deficit. Therefore, our results demonstrate the existence of an unequal neuropsychological profile of visuospatial perceptual dysfunctions and complex visual skills, which would be findings of a CVD.
These disorders are not severe visual impairments, but they do merit studies, early diagnosis and follow-up in order to assess and guide these disabilities. Therefore, neurological injuries due to neonatal hypoglycemia will have an impact on cortical visual impairment, but also to some degree on neurological development and cognition.
We could also conclude that early brain injury from hypoglycemia interferes with the functioning of visual subsystems in particular, yet leaves other subsystems intact and functioning within the normal range.
Ophthalmology, pediatric radiology and electrophysiology service of the Vall d´Hebron university hospital that made possible the studies carried out on the study patients.
The authors declare that there are no conflicts of interest.

©2022 Fonseca-Santos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.