Journal of
eISSN: 2373-6410


Case Report Volume 9 Issue 4
Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Bangladesh
Correspondence: Bipin Chaurasia, chief resident, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh, Tel 8801732531039
Received: April 24, 2019 | Published: July 12, 2019
Citation: Co-existence of arterio-venous malformation and saccular bifurcation aneurysm of a 48years old patient, presented with massive intracranial hemorrhage: case report190Copyright:©2019 Ansari et al.Citation: Ansari A, Rahman A, Ahmed N, et al. Co-existence of arterio-venous malformation and saccular bifurcation aneurysm of a 48years old patient, presented with massive intracranial hemorrhage: case report. J Neurol Stroke. 2019;9(4):189-194. DOI: 10.15406/jnsk.2019.09.00375
Background: Hemodynamic aneurysm (HA) associated with arterio-venous malformation (AVM) is considered as one of the risk factors for intracranial hemorrhage.1,2 Aneurysm appears as a consequence of disruption in the cerebral auto regulatory mechanism which is induced by AVM. According to the previously reported literature, untreated HA after AVM exclusion can regress, remain unchanged, grow or rupture.1–4
Aim of the work: The aim of this case report is to describe a rare case of simultaneous presence of cortical arterio-venous malformation and saccular bifurcation aneurysm of a 48years old patient for which he underwent clipping of aneurysm and total excision of AVM in two sitting within very short interval of time and achieved favorable outcome.
Case report: A 48-year-old normotensive, non-diabetic male presented with sudden onset of severe headache followed by loss of consciousness. After thorough radiological evaluation, he diagnosed as a case of right sided MCA bifurcation aneurysm and small cortical parietal AVM. At first sitting, he underwent right sided pterional craniotomy and clipping of the aneurysm. 5days later he underwent right parietal craniotomy, evacuation of hematoma and total excision of AVM. His post-operative period was satisfactory and was released from hospital without having any neurological deficit.
Conclusion: Proper pre-operative work up, including angiogram should be done for any suspected vascular lesion. Considering the management of our reported case, Author recommends the simultaneous treatment of AVM and HA, which would be most beneficial to the patient.
Keywords: aneurysm, arterio-venous malformation, hemodynamic instability
AVM, arterio-venous malformation; CT, computed tomography; GCS, glasgow coma scale; HA, hemodynamic aneurysm; IEL, internal elastic lamina; MCA, middle cerebral artery; MRI, magnetic resonance imaging
The co-existence of Hemodynamic aneurysm (HA) and Arterio-venous malformation (AVM) is frequently reported in the previous literature by several authors.2–9 Several theories have been proposed to explain the pathogenesis of these dual pathologies and classified them according to the topographic relationship of the aneurysm with the nidus Table 1. Perata et al.2 showed that HAs are mostly present on AVM feeding vessels rather than on other intracranial vessels (11.2% vs. 0.8%). HA is considered as an acquired pathology which serves as a marker of hemodynamic disturbances induced by AVM nidus. The natural history of these dual pathology showed that, aneurysms over time can regress, remain unchanged, can grow, even rupture or may appear at new locations as well.8 In our reported case, patient had cortical AVM with proximal flow related saccular aneurysm, for which he underwent clipping of the aneurysm in the first sitting followed by excision of AVM with evacuation of the hematoma in the next sitting and achieved favorable outcome.
History and physical examination
A 48years old, normotensive non diabetic male presented with sudden onset of severe headache followed by loss of consciousness for 4 hours. He was immediately hospitalized. Admission GCS was E2V2M4. There was anisocoria, whereas right pupil was 5mm, sluggish reacting to light. Left pupil was 4mm and normal reacting to light. Plantar response was bilaterally extensor. However, vital signs were within normal limit. Other systemic examination revealed no abnormalities. This 39-year-old man appeared to be perfectly.
After hospitalization, an urgent CT scan of brain did, which revealed massive hematoma occupying the right sylvian fissure and adjacent parietal lobe (Figure 1). MRI of brain showed T1WI heterogeneously hyper intense lesion present within the right sylvian cistern and adjacent temporo-parietal area. The lesion had multiple intrinsic flow voids in T2WI (Figure 2). Due to initial presentation as intracranial hemorrhage and presence of multiple flow voids in T2WI, a CT angiogram of cerebral vessels was done to sort out the pattern of vascular pathology. CT angiogram showed a saccular aneurysm at the bifurcation zone of right MCA (Figure 3A). After successful clipping of the aneurysm, repeat CT angiogram with 3D reconstruction sequence revealed AVM, supplied by angular artery of right MCA (Figure 3B).

Figure 1 CT scan of brain, axial section showing massive hemorrhage at the right parietal lobe with compression in the lateral ventricle and midline shifting.
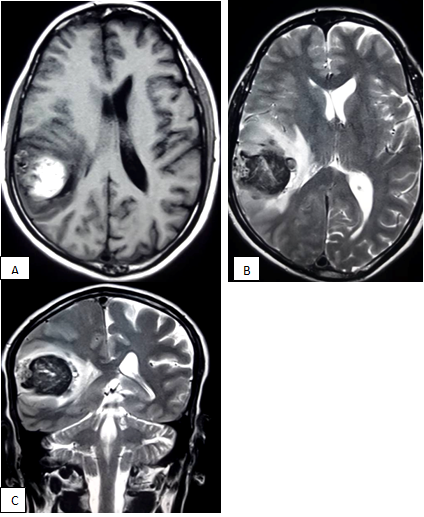
Figure 2 MRI of brain , T1WI axial section showing a mixed intensity lesion within the parietal lobe causing compression of lateral ventricle (A); lesion become heterogeneously hypointense with multiple flow voids (B,C).
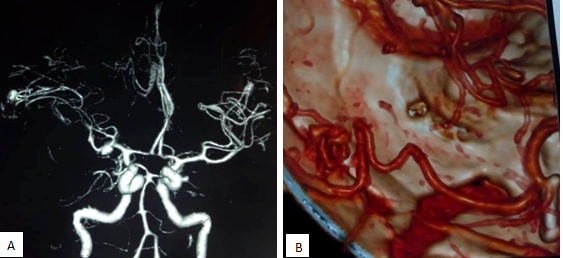
Figure 3 Pre-operative CT angiogram showing right sided MCA bifurcation aneurysm (A); Post-operative CT angiogram showing obliteration of the aneurysm after clipping and a small AVM feeded by angular artery(B).
Operative procedure
Clipping of the aneurysm: With all aseptic precaution, patient placed in supine position with head elevated 30 degree and rotated 30 degree towards left. A standard pterional craniotomy done with flushing of the temporal base. Dura incised in curvilinear fashion, base directed towards orbit. After dissection of the sylvian fissure, aneurysm identified at the bifurcation zone. After that, dissection of the neck of aneurysm done and a permanent straight titanium aneurysm clip applied into the neck (Figure 4A). Careful inspection done to rule out the accidental entrapment of the perforators. Closure done in standard fashion. Patient electively shifted to ICU for proper post-operative management.

Figure 4 Per-operative photograph showing clipping of the aneurysm neck (A); identification of the location of hematoma through brain cannula (B).
Excision of AVM and evacuation of hematoma: Due to failure of clinical improvement, CT scan done at 3rd post-operative day which showed persistence of hematoma with perilesional edema and gross midline shifting. After proper counseling of the patient party, patient had to be prepared for next operation. At 2nd sitting, right parietal craniotomy done. Dura incised in ‘U’ shaped fashion, keeping the base directed towards the superior sagittal sinus. A brain cannula introduced through the crown of the gyrus, through which altered blood was drained. Corticotomy followed by evacuation of hematoma done. Copious saline irrigation given. AVM was identified and total excision done following the surgical principle of AVM surgery. After ensuring hemostasis, closure did maintaining the anatomical plane (Figure 4B).
Post-operative period: Patient again shifted to ICU after 2nd operation. However, by this time, his post-operative period was uneventful. During the time of discharge his GCS was 15. Resected specimen was sent for histopathological examination and biopsy was compatible with Arteriovenous Malformation. Follow up CT scan of brain after 6 months showing Encephalomalacic changes with complete resolution of hematoma (Figure 5). Patient had no neurological deficit and returned to work (Figure 6).
Arteriovenous malformation hemorrhage is responsible for long-term neurological morbidity and
mortality as 35% and 29% respectively.1
When arterio-venous malformation (AVM) is associated with hemodynamic aneurysm, it is considered as an important risk factor for intracranial hemorrhage 2-7. There is wide variation in the literature denoting the prevalence of AVM-associated aneurysms (range 2.7%–58%), but a range of 10%–20% found in the largest case series.7–10 Though there is no sex predilection of the incidence of these dual pathology; however, women are more prone to present with hemorrhage. The average age of presentation is 40years.11,12 The yearly risk of hemorrhage for an unruptured AVM is 2%–4%. The mortality rate after the first hemorrhage is about 10%, and the risk of major disability is 20%–30%.13,14 Brown et al. reported hemorrhage rates as high as 7% per year at 5 years for patients with coexisting AVMs and aneurysms, compared with 1.7% hemorrhage rate per year for patients with AVMs alone1.
Several authors have categorized the aneurysms associated with AVMs based on their topographic relationship with the AVM nidus.15 They can be divided into four groups: unrelated dysplastic or incidental, flow related on proximal feeding vessels, flow-related on distal small feeding vessels and intra tidal (Figure 7) (Table 1). Aneurysm formation is the result of disequilibrium between hemodynamic stress and the condition of the IEL and the intima.16 Hemodynamic stress predisposes the remodeling, degeneration and loss of the IEL. Intranidal aneurysms should be considered “false aneurysms” caused by nidus rupture rather than aneurysm rupture. Proximal flow-related aneurysms located in a main intracranial vessel, up to its primary bifurcation, without IEL disruption and having benign course. Because small arteries involved in arteriovenous malformation are deficient in the smooth-muscle layer,17 the distal flow-related aneurysm lacks IEL and smooth-muscle layer which predisposes for bleeding. They are located at midpoint or distally on a direct AVM feeder. Unrelated aneurysms are assumed to be acquired lesions caused by a combination of hemodynamic stresses (luminal factors) and defective vessel wall.18 AVMs coexisting with HA have a linear relationship with hemorrhage, older age, and infratentorial AVM location.19–21 The presence of a flow-related or an intranidal aneurysm appears to be associated with an increased risk of hemorrhage, both at initial presentation and recurrence. When specifically analyzing flow-related aneurysms, the evidence of association between those lesions and hemorrhage risks is strongest with distally located lesions.
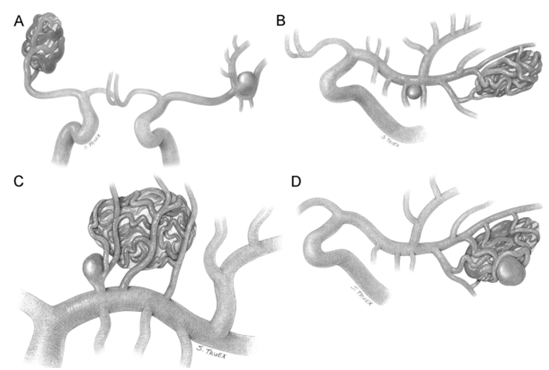
Figure 7 Classification of AVM associated with aneurysm showing unrelated aneurysm (A); proximal flow related (B); distal flow related (C) and intranidal types (D).
Type |
Nature |
Pathological features of vessel wall |
No. of patients |
Clinical presentation |
Treatment modality |
Intranidal |
Pseudo-aneurysm |
Disruption of IEL |
12 |
6 hemorrhage |
Onyx embolization |
Distal flow related |
Saccular, arising from arterial trunk |
Disruption of IEL, |
9 |
7 hemorrhage |
The aneurysm and |
Proximal flow related |
Segmental ectasia |
Stretched/fragmented IEL; no luminal thrombus |
14 |
3 hemorrhage |
Coiling for |
Unrelated |
Saccular, arising from circle of Willis |
Disruption of the IEL |
11 |
4 hemorrhage |
Simply coiling or stent assisted coiling |
Table 1 Summary of Types of Aneurysms Coexisting with AVM30
Three main theories have been proposed to explain the association between AVMs and HA. According to Paterson and Mckissock, increased blood flow through the arterial feeders is responsible for the formation of the aneurysm.22 Anderson and Blackhood thought that this association is part of the existence of multiple vascular anomalies in the same individual.23 Boyed-Wilson stablished the theory that this association are nothing but the incidental finding after thorough evaluation regarding vascular anomalies.24
The goal of operative intervention in all AVMs should be the complete surgical excision of the nidus, as documented by postoperative angiography. Only total surgical excision of AVM or complete embolization of the nidus can completely avoid the risk of subsequent hemorrhage or progression of ischemic neurological deficits.25 Suitable candidates are categorized preoperatively by Spetzler Martin Grade. In the specific cases of AVMs associated with HAs, several factors should be taken into consideration before recommending treatment of these dual pathologies. The microsurgical techniques to excise the nidus of AVM is same following the principle of AVM surgery. Adequate exposure of the surgical field is of paramount importance. The surgeon must have access to the nidus, its feeding arteries, and draining veins while applying minimal brain retraction. Patients presenting with hemorrhage secondary to AVM rupture are rarely managed surgically during the acute phase. Delaying surgery allows the patient to recover from the initial hemorrhage and facilitates hematoma resolution which later on, makes ease the dissection planes between the hematoma and the surrounding parenchyma. Occasionally, the AVM presentation is of a comatose patient with a large cerebral or cerebellar hematoma requires emergency evacuation. In that situation, the goal is to evacuate the hematoma and reduce the acute mass effect or hydrocephalus, leaving the AVM intact. Whenever feasible, however, definitive AVM resection should be deferred for 4–6weeks.26. Comparing the scenario of previously reported literature, our reported case underwent parietal craniotomy followed by evacuation of hematoma and excision of AVM in the same sitting due to failure of neurological improvement after 1st operation.
Although feeding-artery aneurysms less than 5 mm in diameter have been reported to regress after treatment of AVM in some cases,27 they may rupture during or after AVM treatment. Given the concern about possible rupture, endovascular coiling of the arterial bifurcation aneurysm before AVM embolization should be performed.28,29 Though it is recommended for proximal flow related aneurysm, we went for surgical clipping for our case because of its accessible location. The distal flow-related aneurysm is often wide-necked, and may be difficult to clip. In such cases, surgeon can go for Onyx embolization.30,31 Spetzler et al. have advocated that Grade IV and V AVMs should be managed conservatively.9 However, in the presence of associated aneurysms, it is recommended that the aneurysm should be treated with or without intervention to the high grade AVM.
Patients with both AVM and HA tend to have higher hemorrhage and re hemorrhage rates, thereby possessing more aggressive clinical course. Topographic assessment of the aneurysm with the AVM nidus showed that flow related and intranidal aneurysm carries increased rate of intracranial hemorrhage when compared with the unrelated type. So, it is the Author’s recommendation that the treatment should be directed towards the aneurysm first because of its increased rerupture risk followed by excision of the AVM after resolution of hematoma which minimizes the morbidity and mortality.
None
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest.
An informed written consent was obtained from the patient.

©2019 Co-existence, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.