Journal of
eISSN: 2373-6410


Case Report Volume 12 Issue 6
1Senior Consultant Neurology, Pushpawati Singhania Research Institute, India
2Senior consultant & HOD, KINS Institute of Neurosciences, India
3Senior Professor & head- Neurology Unit, University SMS Medical College, India
Correspondence: Dr Kadam Nagpal, MD(Medicine), DM (Neurology), FEBN, FRCP(Edinburgh); Senior Consultant Neurology, Pushpawati Singhania Research Institute, Address: C-86 South Extension Part-II, New Delhi, India-110049
Received: November 12, 2022 | Published: December 22, 2022
Citation: Nagpal K, Prakash S, Jain RS. Anti LGI-1 positive encephalitis in an octogenarian: a curious case of recurrent hyponatremia in elderly- a case report. J Neurol Stroke. 2022;12(6):206-208. DOI: 10.15406/jnsk.2022.12.00530
LGI-1 is mainly expressed in the limbic areas involving temporal cortices and hippocampus. Anti -LGI-1 encephalitis is an autoimmune encephalitis which manifests with cognitive impairment, seizures which are mostly faciobrachial dystonic type and recurrent hyponatremia. We present an 82 year old octogenarian with similar such presentation with recurrent episodes of hyponatremia and cognitive decline having an excellent response to immunotherapy. There has been no mention of a similar presentation in this age group in literature, to the best of our knowledge.
Keywords: Anti LGI-1 encephalitis, refractory status epilepticus, elderly, hyponatremia
Leucine-rich glioma-inactivated-1 (LGI-1) antibody encephalitis is a rare autoimmune voltage-gated potassium channel complex (VGKC) antibody-associated limbic encephalitis. It is classified as an antineuronal surface antigen- or antisynaptic protein-associated autoimmune encephalitis.1 It is second most frequent type of autoimmune encephalitis after anti-N-methyl-D-aspartate receptor encephalitis.2 Common symptoms of limbic encephalitis are cognitive impairment, seizures, and psychiatric disorders, this disease is also associated with faciobrachial dystonic seizure (FBDS) and refractory hyponatremia.3 Unlike other limbic encephalitides, LGI-1 antibody encephalitis is rarely accompanied by tumors4 and shows a good response to immunotherapy.5 There have been several studies in the recent past wherein the mean age group of patients getting afflicted with Anti LGI-1 encephalitis was around 64 years of age and in the past the oldest patient reported was 73 years old.6 However, our patient was an 82-year old octogenarian, with a presentation of frequent hospital admissions due to recurrent hyponatremia and progressive cognitive decline with excellent response to immunotherapy.
Patient 82 year female came with unresponsiveness with episode of seizure (semiology generalized tonic- clonic type) for last 1 day duration. In the background, she had a history cognitive decline which progressed rather rapidly. The cognitive decline primary involved short term memory impairment with difficulty in naming and recognition. She became withdrawn and had apathetic behavior, which had become evident in last 2 months. There was a history of a similar such episode occurred in the preceding week when she was diagnosed with low sodium levels and after the sodium correction was done, patient had a complete recovery. There was no history of fever, head trauma, loss of body weight or decreased appetite. No history suggestive of stroke or any seizure prior. No significant family history. On examination, she was delirious, moving all four limbs spontaneously without any focal deficit. No signs of meningeal irritation were there either. Her routine blood investigations revealed low sodium levels (serum Na-120meq/l) which prompted for slow correction. Her Electroencephalograph (EEG) was normal but her MRI brain (Figure 1 A & 1B) revealed left hippocampal edema with underlying age related cortical atrophy, which was assumed to be post ictal edema. However, patient was planned for a lumbar puncture which the attendants denied, as the patient got better after correction of sodium levels and they didn’t want to pursue any invasive testing at this age insisted for a discharge. But only within 10 days, she got readmitted with worsening of sensorium and episodic and twitching movements of right side of face that was followed by twisting movements of right arm which were associated with simultaneous lip smacking movements. These episodes became frequent and patient was having around 10-12 such episodes every day. There was no history of fever or headache or any head trauma prior. Her routine parameters again showed low sodium levels (serum Na-126 meq/l). Another MRI brain was conducted which showed nearly symmetrical bilateral medial temporal hyperintensities on T2 weighted /FLAIR and diffusion weighted images (Figure 1C-1F). Subsequently, a lumbar puncture was done which showed slightly elevated CSF proteins (90.62 mg/dl), glucose (62 mg/dl) and 4 cells. The autoimmune encephalitis panel was sent, which returned positive for Anti-LGI-1 antibodies in both serum and CSF samples. She was treated with immunoglobulins and intravenous pulse methylprednisolone simultaneously and multiple antiepileptic drugs were started in quick succession as the seizures became refractory and there were episodes non convulsive status epilepticus. Electroencephalograph (EEG) showed runs of intermittent generalized epileptiform discharges (Figure 2). Her seizures were eventually controlled on Levetiracetam, Lacosamide, Phenytoin and Perampanel in appropriate doses. Whole body FDG PET CT scan was suggestive of inhomogenously increased FDG uptake involving bilateral basal ganglia and bilateral medial temporal lobe (Left>Right) with absence of FDG avid disease elsewhere in the body (Figure 3A-3F). Thereafter patient was administered Injection Rituximab at 1g once every two weeks preceded by premedication. During her course of treatment she responded dramatically in terms of cessation of seizures and cognition was significantly better as compared to previously. But unfortunately, she didn’t come for her subsequent follow up visits.
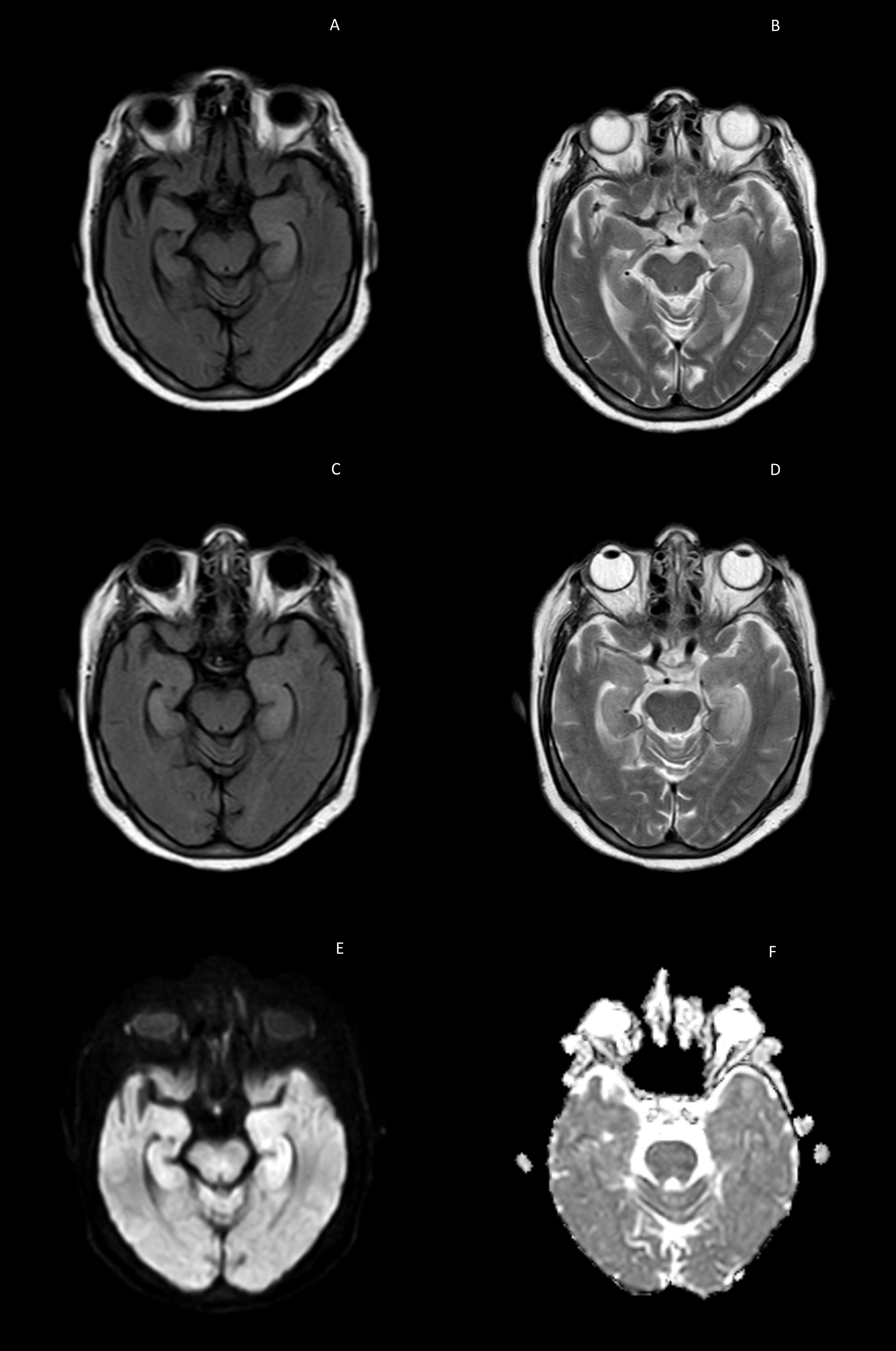
Figure 1A & 1B T2 weighted and FLAIR images suggestive of left sided medial temporal hyperintensities.
Figure 1C & 1D T2 weighted and FLAIR images suggestive of bilateral bulky medial temporal lobes with increased signal / hyperintensities seen on subsequent scan.
Figure 1E & 1F DWI hyperintensities seen in median temporal lobes with no hypointensity on ADC images suggestive of vasogenic edemae.
LGI-1 is mainly expressed in hippocampus and temporal cortex where it is secreted in synaptic space. The clinical manifestations of anti-LGI-1 encephalitis include seizures, cognitive impairment, psychiatric disorders and refractory hyponatremia with peripheral nerve hyperexcitability. Most common type of seizures are focal seizures, which are seen in upto 81.4% patients.7 While a significant proportion present with faciobrachial dystonic seizures, which is seen in upto 35.9-47%.8 Recognition of seizures with MRI findings showing signal signal changes in temporal lobes are clue to early diagnosis. The diagnosis is confirmed with a positive LGI-1 antibody in serum and cerebrospinal fluid sample. Tumors are rarely found in Anti LG-1 encephalitis, however association of thymoma and lung carcinoma have been reported.9 In view of similar clinical presentation, Anti LGi-1 encephalitis has to be distinguished from viral encephalitis, Hashimoto’s encephalopathy, Creutzfeldt Jacob disease and other forms of autoimmune disorders. Often upto 90% of cases have favourable responses to immunotherapy in terms of seizure control, however amnesia or dyscognitive changes may last for months. Relapses are often seen in upto 18%of cases, therefore longer follow ups are of paramount importance.10
Often in an octogenarian patient, recurrent hyponatremia is considered as hyponatremia of elderly and progressive cognitive is regarded as senile dementia. However, this case highlights in an appropriate clinical context there should be a high index of suspicion for autoimmune etiology, regardless of the age of patient. And furthermore once the diagnosis of autoimmune etiology is established, there should be no delay in initiating the immunotherapy including the second line agents, because the clinical outcomes may be highly rewarding.
None.
The authors declare no conflicts of interest.
None.

©2022 Nagpal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.