Journal of
eISSN: 2373-6410


Case Report Volume 4 Issue 5
Department of Neurosurgery, Azienda Ospedaliero-Universitaria ?OO-RR? Foggia, Apulia, Italy
Correspondence: Rizzi Gaetano M.D, Department of Neurosurgery, Azienda Ospedaliero-Universitaria OO-RR Foggia, Apulia, Italy, Tel +39 3394010533, Fax +39 0881732487
Received: December 21, 2015 | Published: April 1, 2016
Citation: Rizzi G, Berardi A, Bozzini V, Donnarumma P, Merlicco G (2016) A Rare Case of Cavernous Angioma of the Cauda Equina with SpinalSubarachnoid Hemorrhage in a Patient with Multiple Brain Cavernomatosis. Case Report and Review of Literature. J Neurol Stroke 4(5): 00140. DOI: 10.15406/jnsk.2016.04.00140
Objective: To report a rare case of cavernous angioma of the cauda equina with signs of intratumoral and subarachnoid hemorrhage in a patient affected by multiple brain cavernomatosis, and to discuss the clinicoradiological and surgical features of these uncommon lesions using the data available in the literature.
Summary of Background Data: Cavernous angiomas of the cauda equina are extremely rare lesions, accounting for only 5% to 12% of all vascular lesions of the spine, and rarely cause spinal subarachnoid hemorrhage. Including the present, only 27 cases are reported in literature.
Methods: A case report and review of the literature.
Results: A 50 year-old female with history of multiple brain cavernomatosis, presented with low back pain, paraparesis and lower limb hypoesthesia from six months. MRI scans showed a well defined intradural extramedullary mass in the cauda equine at L2 level, with signs of subarachnoid hemorrhage in L5-S1. An “en-bloc” removal of the lesion was performed. The patient was discharged with no neurological deficit. MRI scan 6 months after surgery showed total excision of the lesion with no recurrence.
Conclusion: Cavernous angiomas of the cauda equine associated with multiple cerebral cavernous angiomas are extremely rare lesions. They may present low back pain, sciatica, neurologic deficit and subarachnoid hemorrhage. They can be successfully surgically removed in order to prevent bleeding and dangerous enlargement of the lesion.
Keywords:Cavernous angioma, Cavernoma, Cauda equine, Subarachnoid hemorrhage, Spinal cord
Cavernous angiomas of the spinal cord are extremely rare lesions representing only 5% to 12% of all vascular lesions of the spine.1 Their location, in intradural extramedullary space and their coexisting with multiple brain cavernomatosis is sporadic.2 We report a case of a cavernous angioma of the cauda equina with intralesion and spinal subarachnoid hemorrhage in a patient with multiple brain cavernomatosis. A review of the literature is also conducted.
A 49-years-old woman was admitted to the department of Neurology of our hospital for drug-resistant headache on August 2013. An MRI scan showed multiple millimetric lesions located in temporal, occipital left lobe and in the pontomesencephalic region (Figure 1). The patient was discharged one week later with a diagnosis of multiple brain cavernomatosis. One year later, on August 2014, she consulted for low back pain, bilateral sciatica and paraparesis from six months. The neurologic examination showed lower limb hypoesthesia with no clear radicular distribution, absence of rotulus, achilles and plantar reflex. A brain CT scan showed the stability of the lesions. A lumbar MRI scan showed 2.1 cm intradural-extramedullary lesion at L2 level. The lesion appeared hyperintense on T1-weighted images with no gadolinium enhancement, and hypointense in long TR sequences. An anomalous enhancement of a cauda equina nerve root and the presence of subarachnoid hemorrhage (SAH) at L5-S1 were also evident (Figure 2-3). The patient underwent surgical excision of the lesion. In the prone position, a L1-L2-L3 laminectomy was performed and dura opened in the midline under microscope magnification. A 2.1 x 2 cm dark-blue mass was exposed. The lesion was surrounded by the cauda equina nerve roots and was closely adherence to a single root (Figure 4). After root sacrifice, an “en bloc” removal was performed. Histopathologic examination showed a large pseudocystic space containing blood and numerous blood vessels with thin walls, lined by flat endothelium. The picture was suggestive of a cavernous angioma with intralesional hemorrhage (Figure 5-6-7). The post-operative course was free from complications and the patient discharged six days after surgery. At six months follow-up the paraparesis and sensory deficits improved and MRI scans showed complete removal of the lesion and no recurrence. (Figure 8). The patient is healthy and brain cavernomatosis following-up.
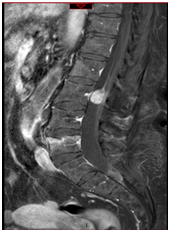

Figure 2&3 Preoperative sagittal (Figure 2) and axial (Figure 3) T1 weighted MRI scan after gd enhancement, showing heterogeneous hyperintense intradural cavernous angioma at L2 level.
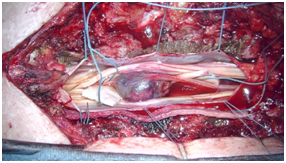
Figure 4 Intraoperative photographs showing a 2.1 x 2 cm dark-bluish mass was exposed closely adherent to a single root nerve.
Cavernous angiomas of the spinal cord are vascular malformations, which account for approximately 5 to 12 % of spinal cord lesions.1,3,4 The occurrence of intracranial and intraspinal cavernous malformations in the same patient has been reported, but is quite rare.3 The first report of multiple brain cavernomatosis was published in 1899 by Ohlmacher, who found three brain cavernomas in an autopsy performed on a patient with intractable epilepsy.5 The first published case of a spinal cavernoma was by Hadlich6 in 1903, who revealed the lesion in the spinal cord during autopsy. Mutations in at least three genes, KRIT1 (also known as CCM1), CCM2, and PDCD10 (also known as CCM3), cause familial cerebral cavernous malformations. The precise functions of these genes are not fully understood. Studies show that the proteins produced from these genes are found in the junctions connecting neighboring blood vessel cells. The proteins interact with each other as part of a complex that strengthens the interactions between cells and limits leakage from the blood vessels. Mutations in any of the three genes impair the function of the protein complex, resulting in weakened cell-to-cell junctions and increased leakage from vessels as seen in cerebral cavernous malformations.7,8
It is accepted knowledge that cavernous angiomas malformation of the cauda equina occurs in the nerve root, the interior side of dura mater and pial surface of the spinal cord within the dura mater outside of the spinal cord.9 Spinal Schwannoma and cauda equina ependymoma, as well as other spinal intradural extradural space-occupying lesions,10 should be considered in the differential diagnosis. MR imaging is helpful and can be diagnosed if the presence of blood and its breakdown products in various stages are suggested by various intensity signals within the lesion.11
Slow-progressing symptoms are the most common mode of presentation. The pathological process that causes slow progression is not entirely clear. The mechanism probably involves repeat microhemorrhages, the gliosis neurotoxic effect of hemosiderin, impaired microcirculation due to local pressure, and progressive enlargement of the lesion.12
The median age of symptom presentations is 43.3, with a male predominance. With the present, to date only in one case co-existance of brain cavernomatosis has been reported.13
Typically, symptoms include low back pain and sciatica, neurological deficits, or a subarachnoid hemorrhage. In the twenty-six cases reported to date in the literature, the clinical symptoms were related to SAH in four cases,14-16 compression of nerve roots in 15 cases,10,15,17-20 not mentioned in five cases, ICTH in one case and ICTH +SAH in one case.5,11,12,19-22 In our case, the symptoms were related to the compression of neural structures and not to the presence of subarachnoid hemorrhage.
Total “en-bloc” removal is the gold standard for treatment. It must be performed under microscope magnification in order to spare the root nerve closely adherent to the tumor. The nerve root merging into the tumor can be cut without additional neurological deficits.11,5,19,21
Cavernous angiomas of the cauda equine, in particular in association with cerebral cavernous hemangiomas, is an extremely rare condition that may present low back pain, sciatica, neurologic deficit and subarachnoid hemorrhage. It can be successfully treated by surgery in order to prevent bleeding and dangerous enlargement of the lesion.
None.
None.

©2016 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.