Journal of
eISSN: 2373-6410


Research Article Volume 8 Issue 1
Department of Neurosurgery, Al- Azhar University, Egypt
Correspondence: Mohamed Hasan Mansour, Neurosurgery Department, Faculty of Medicine, Al- Azhar University, Egypt
Received: November 11, 2017 | Published: January 5, 2018
Citation: Mansour MH (2018) Which is Superior in Diagnosing Focal Brain Lesions: CT, MRI, MRS or Histopathology? J Neurol Stroke 8(1): 00271. DOI: 10.15406/jnsk.2018.08.00271
Introduction: In spite of recent advances in the used preoperative diagnostic tools, there is no single accurate diagnostic one and none of them had a hundred percent accuracy. So, misdiagnosis still occurs. Many trials were done to evaluate the accuracy of these tools individually or in combination.
Patients and Methods: This study was conducted over the last three years in Al-Azhar university hospitals (Neurosurgery, radiology and histopathology departments) on 51 patients out of total 100 patients suffering from focal brain lesions as diagnosed by CT and/or MRI brain. All of them were requested for MRS before planning for biopsy and histopathology. Fifty patients of them were operated for biopsy and histopathology either by minimal invasive (stereotaxy) or invasive open craniotomy. Mean age was 54 years. Female/male ratio was 2/3. The most frequent symptom was headache. The decision to operate was made if still no accurate final diagnosis and /or progressive deficits.
Results: MRS was supporting to biopsy in 45 cases, while biopsy was mandatory after MRS in 5 cases and MRS was beneficial in avoiding unnecessary biopsy in 2 cases.
Conclusion: Both MRS and histopathology are complementary in diagnosing focal brain lesions. MRS must be ordered before biopsy and must be included in any tumor grading.
Keywords:Focal brain lesions, MRS, Biopsy, Histopathology
Diagnosis is the key for treatment plan. Some fundamental tasks for accurate diagnosis include: (1) Anatomic localization of the lesion and its relation to surrounding structures which could be determined by CT and /or MRI brain. (2) Evaluation of the activity of this lesion (hyperactive, normal, or silent) by magnetic resonance spectroscopy (MRS). (3) Determining cell of origin of the lesion through tissue sampling which is the gold standard for diagnosing the type of cellular elements of the lesions but taking in consideration that partial excision of tumor tissue may change the behavior of the tumor. Other fundamental tasks like the clinical condition either local or general cannot be neglected. When a patient comes for the evaluation of a focal brain lesion, it is often difficult to differentiate between tumoral and non-tumoral brain lesions and frequently creates a dilemma for physicians and surgeons for further management. The current gold standard characterization of a brain tumor is by histopathological analysis.1
Histopathological analysis is the standard procedure routinely used to reveal the amount of necrosis, proliferative regions, collagen and vascularity within the tumor area.2 The introduction of MRS has directed the attention of neurosurgeon to use it as a noninvasive tool to raise the preoperative diagnostic accuracy, which may obviate the necessity for open surgery especially at eloquent parts. Magnetic Resonance Spectroscopy (MR Spectroscopy) is one of the tools used to determine the molecular structures of compounds or to detect the compound presence. MR Spectroscopy provides metabolic information from living brain. The major brain metabolites detected are choline, creatine, N-acetyl aspartate (NAA), lactate, myo-inositol, glutamine, glutamate, lipids and the amino acids leucine and alanine.3 This is the substrate of MRS, So that Magnetic Resonance spectroscopy brain lesions show abnormal values of these metabolites as compared to normal tissue. (MRS) is non-invasive sensitive, however, relatively nonspecific modality in differentiating neoplastic and non-neoplastic brain lesions.4
There is strong evidence for a reduction in Nacetylaspartate and increase in choline containing compounds in tumor relative to normal brain parenchyma. Also, for some metastatic lesions and for high-grade gliomas, typically, there are resonances corresponding to lactate or lipids.5 MR Spectroscopy is a potential tool for differentiating neoplastic from non-neoplastic brain lesions.6 So that the goal of this study is to describe the role of Magnetic Resonance spectroscopy (MRS) among other diagnostic tasks for focal brain lesions and its relationship to histopathology.
The study was conducted in departments of Radiology, Neurosurgery and Histopathology Al-Azhar university hospitals, Cairo, Egypt, from May 2011 till March 2014 and included 100 patients with focal brain lesions as shown by CT and /or MRI brain. Clinical evaluation included a detailed history and physical examination.
The following procedures were used routinely for the diagnosis:
Clinical evaluation: general and neurological examination after detailed clinical history was done for all patients. Weakness was the Most common clinical presentation (30%) followed by headache (24%).
Anatomic studies (CT and /or MRI brain): for one hundred patients suffering from neurological deficit, CT brain and MRI brain was done to pick up any lesions and to determine its anatomic location with comparison of the sensitivity and specificity of both.
Biological studies to evaluate the activity of the lesion by MRS: only for fifty two patients to evaluate the metabolites of brain tissue.
Histopathological studies: To evaluate the morphological changes of tissues, with comparison of diagnostic accuracy of MRS and histopathology.
Follow up of the patients to evaluate the diagnostic modality: the final diagnosis may be changed by therapeutic diagnosis (only in one case).
All the patients found to have focal brain lesions on conventional MRI sequences were included in the study. MR Spectroscopy (through single voxel technique) was performed in all these patients after informed consent. Initially, post contrast conventional MR imaging was done to localize the lesion and then voxel was placed on volume of interest. After water suppression, a point-resolved spectroscopy (PRESS) technique was used for localization and the studies were obtained with parameters including TE and TR of 135 and 1500 respectively.
Exclusion criteria for biopsy: Eloquent areas, absence of mass effect, absence of surrounding edema, hypometabolic MRS and regressing lesions in follow up radiology. On the basis of MRS findings, lesions were categorized into neoplastic and non-neoplastic lesions. The spectra were analyzed for the signal intensity of NAA, choline, and creatine and for the presence of lipid and lactate peak. Ratios were manually calculated for Cho/Cr, and Cho/NAA ratio. NAA is a marker of neuronal integrity and peaks at 2.02 ppm. Choline is an indicator of cell turnover and peaks at 3.22 ppm. Similarly, creatine is involved in cell metabolism and peaks at 3 ppm. Lipid and lactate peak are normally absent in brain tissue and when present peak at 1.33 ppm and may overlap each other. Neoplastic lesions have elevated choline and reduced
NAA peaks on MR spectrum with increase choline /creatine and choline/ NAA ratios. Reduced Cho, Cr and NAA peaks on MR spectrum are suggestive of non-neoplastic lesions. Cho/creatine and choline/NAA ratio are usually not altered in nonneoplastic lesions. Lipid and lactate peak can be seen in high grade tumours and also in infectious lesions. Lesion was also evaluated on conventional MR sequences before making the final MRS diagnosis. In this study tissue sampling was read by 2 different histopathologists. Data was entered using Statistical Package of Social Sciences (SPSS) program version 16.0. Percent agreement between spectroscopy and histopathology for focal brain lesion in differentiating from neoplastic and nonneoplastic lesion was calculated by using kappa statistic. P value less than 0.05 was considered as significant. Sensitivity, Specificity, positive, negative predictive value and accuracy of MR Spectroscopy was calculated. Comparisons of mean value of neoplastic and non-neoplastic lesions were done by using t-test.
There were 28 (28/52) male patients while 24 (24/52) were females. The mean age was 40 ± 18 years with a range from 4 to 76 years. For males, age range was 6-76 years with mean age 41 ± 16 years while in females it ranged from 4 to 67 years with a mean age of 34 ± 22 years.
Regarding the all one hundred cases, the CT brain was negative in 2 cases (one case of infarction and another case of glioma as confirmed by MRI), and was false in one case (false regarding the location and nature of the pathology). Regarding the fifty two cases in Whom MRS was done, one case was read by magnetic resonance spectroscopy as infarction at mid brain with improvement of the clinical condition of the patient, so that histopathology was avoided in this case. Another one was read by MRS as benign lesion at the tectum of mid brain with hydrocephalic changes so only ventriculoperitoneal shunt was done with follow up of the patient regularly. So that the comparative study was done among 50 cases only out of the fifty two with exclusion of these two cases. Forty nine cases out of the 50 cases, were read easily by MRS (45 as neoplastic lesions and 4 as non-neoplastic lesions), while one case (1/50) was reported as difficult diagnosis between infection or high grade glioma where stereotactic aspiration of the lesion and histopathology confirmed to be pyogenic brain abscess and follow up proved clinical improvement of the patient. The histopathology was not coinciding with the MRS findings in 2 cases. The histopathologically proved neoplastic lesions (n=45) included `anaplasic astrocytoma (n=33), lymphoma (n=3), anaplastic ependymoma (n=2), oligodendroglioma (n=3), and neuroectodermal tumours (n=4).
The histologically non-neoplastic cases (n=5) included granulomatous infection (n = 2), chronic haematoma (n=1), bacterial abscess (n=1) and fungal infection (n=1). 2 cases had different histopathologic diagnosis from that obtained by MRS. One case was read by different histopathologic results, (infarction versus glioma?). Increase Cho/Cr (5.38 ± 6.10) and Cho/NAA (11.33 ± 16.3) ratio were observed in neoplastic lesions as compared to nonneoplastic lesions (Cho/Cr = 2.85 ± 2.47) (Cho/NAA = 3.15 ± 2.67) in this study with significant p-value of 0.005. Similarly, significant increased Choline value (2.51 ± 1.8) mmol/l was noted in neoplastic lesions compared to (1.41 ± 0.73) mmol/l in nonneoplastic lesions with p-value of 0.000. Mean NAA value in neoplastic lesion was (0.45 ± 0.35) mmol/l compared to (0.60 ± 0.29) mmol/l in non-neoplastic lesions with p-value of 0.000. The mean Cr value in neoplastic lesion was (0.73 ± 0.64) mmol/l compared to (0.65 ± 0.44) mmol/l in non-neoplastic lesions with significant p-value of 0.001.
Spectrum was also observed for presence or absence of lactate/lipid peak. Regarding lactate and lipid peak, lipid/lactate peak were observed in 26 spectrums. Of these 18 (41.86%) were noted in 43 neoplastic lesions and 8 (80%) in 10 non-neoplastic lesions. Thus there were 40 true positive, 3 false positive, 7 true negative and 3 false negative results reported on MRS based assessment of likely neoplastic brain lesions. Based on these findings, MR Spectroscopy has a sensitivity of 93.02%, specificity of 70%, positive predictive value of 93.02%, and negative predictive value of 70% and diagnostic accuracy of 88.67%. After calculating the percent agreement by chance alone, kappa statistics was applied which showed a good agreement between MR Spectroscopy and histopathology for differentiating neoplastic and non-neoplastic lesions [k=0.630, p- value, <0.001].
In spite of advances in preoperative diagnostic tools, misdiagnosis still occur, so that seeking for more preoperative diagnostic tools are needed and we can postulate that: diagostic tools are complementary, because Fundamental tasks for accurate diagnosis are, anatomic localization of the lesion and relation to surrounding structures, the activity of the lesion (either hyperactive, normal, or silent) and cell of origin of the lesion. The anatomic location of the lesion and relation to surrounding structures may be determined by CT and /or MRI brain, while the activity of the lesion may be determined by MRS of brain, but tissue sampling is still the gold standard for diagnosing the type of cellular elements of the lesions. So we can say that all of these diagnostic tools are complementary.
Regarding the accuracy of CT in diagnosing focal brain lesions: it was accurate in picking up the location of the lesions in 98% of cases, while the lesion was missed in 2% of cases. Among the picked up lesion, one was pseudopositive regarding the location of the lesion and nature of the pathology (Figure 1), which may be explained by the massive venous engorgement due to the mass effect exerted by the chronic subdural hematoma leading to localized edema according to the compressed vein. This explanation is nearly similar to that explanation of pseudolesion of the non-cirrhotic liver which is due to transient extrinsic compression, typically caused by ribs or the diaphragm, and that due to a "third inflow" of blood from other than the usual hepatic arterial and portal venous sources.7
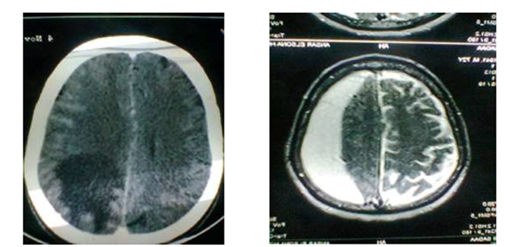
Figure 1 (a):CT with contrast: left parieto occipital deeply seated lesion; (b): MRI OF THE SAME PATIENT: ch. subdural hematoma.
Regarding CT pseudolesions: Intracranial psuedolesions are diagnostic pitfalls that sometimes confuse young radiologists and might deceive experienced radiologists alike. There are two types of intracranial pseudolesions: (1) CT or MRI artifact mimicking a true lesion. (2) Intracranial disease or condition mimicking a different one.8,9 In this study MRI was accurate in picking up the anatomic location of the lesions in 100% of cases. So that MRI brain is more accurate than CT in diagnosing anatomic location of the lesion. We must keep in mind, Conventional MR imaging is a useful tool in the evaluation of tumoral and non-tumoral brain lesions but not really sufficient for diagnosing all conditions.10 MRI was superior in two directions (1) some lesions may be not detected by CT and only can be detected by MRI especially posterior fossa lesions or early infarction. (2) CT may give false localization of some lesions. MRS does not replace conventional MRI but complements it. When augmented with a comprehensive patient evaluation including clinical history and imaging studies MRS is able to detect abnormalities that are invisible to MRI because metabolic abnormalities often precede structural changes. It also provides additional information as a prognostic factor while following the progression of the disease and evaluating the response to treatment.10 CT and MRI are sensitive tools for categorizing brain lesion, but they cannot always distinguish between neoplastic and non-neoplastic brain lesions while MR Spectroscopy can help in differentiating neoplastic from non-neoplastic lesions.11
Regarding the pathologic spectra of MRS: Adamson et al.12 stated that, Cho/NAA ratio greater than 1 was considered to be positive for neoplasms, while Mcknight et al.13 demonstrated that the sensitivity and specificity of Cho /NAA ratio greater than 2.0 (=NAA/Cho ratio less than 1/2) was 96.5% and 70% respectively and ratios greater than 2.5 the sensitivity to detect tumors dropped to 90% but specificity increased to 86%. Although elevated Cho and reduced NAA are found in tumors, these characteristic must be used cautiously as other lesions may exhibit similar features. Non neoplastic lesions that may have tumor –like spectra include inflammation, stroke, multiple sclerosis, radiation necrosis and gliosis.14-16 So that the accuracy of MRS in differentiating neoplastic from nonneoplastic focal lesions is a matter of debate as found also in our study as we demonstrated some cases with Misdiagnosis (Figures 2 & 3) and others without definite diagnosis (Figure 5). But the definite finding or the key of reading MRS is NAA/Choline, which may be less than ½ (hyperactive lesion) or more than ½ (hypoactive lesion) as reported by Majos et al.11 Keeping in mind the spectral pattern of low - grade neoplasms may be similar to that of normal Parenchyma as reported by Lazareff et al.17 and Hwang et al.18 MRS is relatively nonspecific modality in differentiating neoplastic and non-neoplastic brain lesions.4
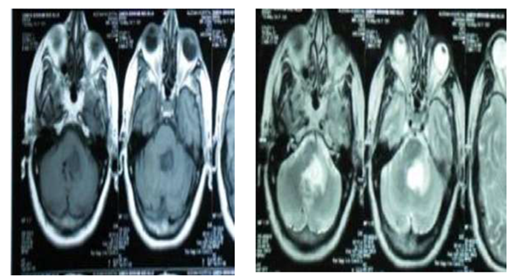
Figure 4a MRI BRAIN: Glioma versus infarction, PATIENT clinical data is only headache with improvement of ataxic gate & she refuse sterotactic biopsy, so that MRS was done.
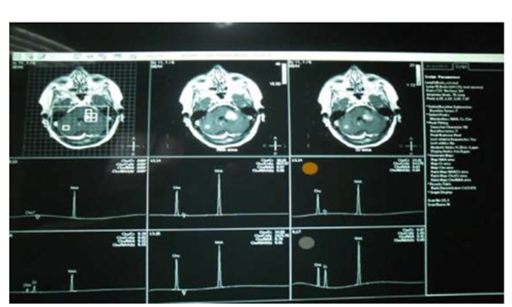
Figure 4b MRS: Hypoactive lesion most probably infarction with regression of the size of the lesion, Clinical follow up: improving (infarction).
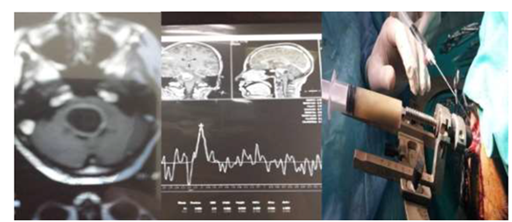
Figure 5 (a) Brain stem cystic lesion of female patient 40 years old with disturbed conscious level. (b) MRS: showing? glioma or infection without definite diagnosis. (c) Stereotactic biopsy: Evacuation of 30 cc of pure pus with improvement of the patient on table.
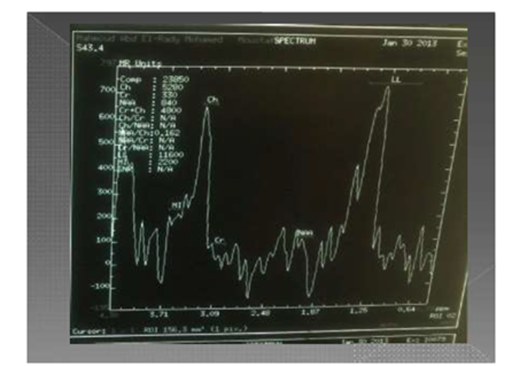
Figure 6 Key of reading MRS = PATHOLOGIC SPECTRA OF MRS =NAA / Choline which may be less than 1/2 (hyperactive lesion) or more than 1/2 (hypoactive lesion).
So that we can postulate that: MRS is good in differentiating lesions into hyperactive, normal, or hypoactive lesions according to NAA/Cho ratio. And this theory is important for surgeons especially in dealing with lesion at eloquant areas (Figure 4). MRS cannot decide the origin of tumor, it only gives hints about the nature of some metabolites denoting hyperactivity or hypoactivity of tumor cells so we can classify lesions spetroscopically into hot (hyperactive) or silent (hypoactive). While the histopathology is considered the gold standard for diagnosing any lesion, there are 2 problems; the first is the site of the biopsy which may be differ from the center to the periphery or outside the lesion (sampling errors). The second is different pathologists reading. To overcome sampling errors, multivoxel MRS has been used successfully to guide stereotactic biopsy, with sampling of higher choline areas, hence greater tumor activity, which is the ideal site for biopsy. If performed in the site where the Chho/NAAis maximum, the biopsy will show high tumor infilteration yielding a high success rate and increased diagnostic cofidence as reported by Martin et al.19 So supporting the postulation of complementation of diagnostic tools.
Despite worldwide efforts to standardize tumor evaluation criteria, the morphological classification of some human brain tumors is subject to interpretation. This uncertainty comes, among others, from the intra and inter- biopsy morphological heterogeneity of tumor tissue. In addition, the descriptive nature of morphology-based histopathology raises some discrepancies among experts.2
Due to the previous common problems of histopathologic diagnosis, we recommend to modify WHO classification of tumors especially gliomas to involve spectroscopic activity of the lesion besides histopathologic grading. Hopping for more advances in diagnostic tools in future, to be similar to finger print for each pathology.
None.
None.

©2018 Mansour. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.