Journal of
eISSN: 2373-6410


Research Article Volume 5 Issue 4
Movement Disorders Research & Rehabilitation Centre, Wilfrid Laurier University, Canada
Correspondence: Quincy J Almeida, Movement Disorders Research & Rehabilitation Centre, Faculty of Science, Wilfrid Laurier University, 75 University Ave. W, Waterloo, N2L 3C5, ON Canada
Received: October 31, 2016 | Published: December 6, 2016
Citation: Almeida QJ, Ersser K (2016) Assessing the Contributions of Optic Flow: Strategies to Improve Gait in Parkinson’s Disease. J Neurol Stroke 5(4): 00182. DOI: DOI: 10.15406/jnsk.2016.05.00182
Visual cues are suggested to be an effective strategy to improve common gait deficits in individuals with Parkinson’s disease (PD), yet we do not fully understand how vision and other forms of sensory feedback aid gait in disordered populations. The present study evaluates whether optic flow is sufficient to improve gait. Two groups were tested in this study: 20 individuals with idiopathic PD “Off” anti-Parkinsonian medications (average=14.7hrs), and 11 healthy age-matched control participants. Participants walked across a computerized carpet in four visual conditions, the first three conditions were at a self-selected pace: i) Normal Vision: walking across the carpet at a self-selected pace with normal vision available, ii) Ground lines: walking while stepping toward lines, iii) Optic flow cues: walking at a self-selected pace while wearing a visual feedback device (the device provides an illusion of moving lines for feet to step towards), and iv) Optic flow plus: attending to an auditory metronome that matched the self-selected pace of the participant (as determined in condition i). Optic flow did not elicit improvements in step length or velocity for the PD participants; only the ground lines improved step length, which concurs with previous studies. Therefore, optic flow alone could not improve normal step lengths in individuals with PD. Only when vision was available did normal stepping occur. Vision is known to compensate for impairments in proprioception. Our results suggest that conscious perception of motion, produced in part by vision and proprioception, is required for improvements in locomotion. Thus we have provided a glimpse as to why optic flow is not effective.
Keywords: optic flow, parkinson’s disease, proprioception, self-motion, vision
One of the most debilitating symptoms of Parkinson’s disease (PD) is deviations from normal gait that hinder mobility. Typically, gait-related symptoms include: stooped posture, shuffling and shortened steps, difficulty changing direction of locomotion, apparent decreased arm swinging, and perhaps difficulty in the initiation of gait. The importance of these symptoms is that individuals with PD may suffer from immobility or increased prevalence of falls. A closer look at spatiotemporal parameters reveals specific impairments in PD including: a reduced stride length, increased time spent in double limb support, decreased velocity of locomotion, and oppressed range of motion at all lower limb joints. Slowness is believed to be the result of reduced speed and amplitude of movement execution.1 Research has theorized that abnormally slow movements in PD are the result of a disordered signal from the basal ganglia; the termination of the signal is inappropriately timed, thereby delaying the execution of the next movement in the sequence.2,3 However, recent research has advocated that motor set function of the basal ganglia may be involved in controlling movement amplitude, and that this is disrupted in PD.4 More specifically, the function of the basal ganglia may be the dynamic modulation of movement execution, processing, integrating or internal generation of self-motion.5˗7
During postural tasks such as walking, compared to normal subjects, individuals with PD are thought to be more reliant on vision.8,9 Studies have shown that compared to self-generated tasks bradykinesia is less evident when individuals with PD use visual cues to improve motor performance.6 In gait specifically, spatial parameters, such as step length, are enhanced by both external visual cues and dopaminergic therapy.7 The gold-standard of visual cueing for improving step length is placement of transverse parallel lines on a walking surface.1,10
Prokop et al.,11 found that optic flow regulates walking velocity primarily because of its effects on step length. Regardless of whether optic flow in the visual field is produced from self-motion or is artificially provided as an illusion, benefits to gait in PD have been observed similarly to other visual cues. Azulay et al.,10 suggest that an important role of vision in the improving stride length is perception of motion. When stroboscopically projected lines were used to suppress dynamic vision, improvements in gait were not observed. One hypothesis is that visual cues help control movement execution by allowing better approximation of a sufficient stride length because of reduced reliance on kinesthetic feedback.4 The mechanisms in the brain that allow for enhancements of gait as a result of visual feedback remain controversial, though there is agreement that higher levels of processing are involved. Visual cues are thought to be effective because the signals produced bypass the basal ganglia in such a manner that they go directly to the occipital lobe and then via fronto-cortical connections to the frontal lobe where each step can be executed normally.1
In previous studies that proposed visual cueing, specifically optic flow in nature, as effective for improving gait parameters, have not separated such cueing from other kinesthetic input to determine its true effectiveness. Isolation of visual feedback was achieved by moving optic flow from the feet to the eyes. The purpose of this study was to elucidate the contribution of optic flow is adequate to increase in the parameters of gait in PD, or if other kinesthetic feedback is the underlying cause.
Subjects
Thirty subjects participated in this study: 20 individuals with Parkinson’s disease (PD) and 11 healthy age-matched controls. Individuals with PD were recruited using a database available at the Movement Disorders Research and Rehabilitation Centre, Wilfrid Laurier University, Waterloo, Ontario, Canada. The mean age of individuals with PD was 67.6 years, with a range between 50 and 79 years. Healthy-age matched control participants were recruited using the Waterloo Research in Aging Participant (WRAP) pool of healthy seniors, or they were the spouses of the individuals with PD. The average age of healthy controls was 68.5 years, with a range of 48 to 78 years. All participants gave informed consent according to the regulations of the university ethics committee of this that approved this study.
Individuals with PD selected for this study were diagnosed as having idiopathic PD by a movement disorders specialist, and did not suffer from: freezing, severe dyskinesias, or postural instabilities that would have prevented them from completing the study. Furthermore, based on participant history, these individuals were known to be responsive to dopaminergic treatment, thus allowing us to confirm that participants were in an “Off” medication state. All participants were confirmed to have no other neurological problems aside from PD, lower limb difficulties, hearing deficits that would influence their ability to attend to the auditory metronome, or any condition that could hinder their ability to see the visual stimulus. Specifically, all participants were asked: if they had a history of neurological problems; if they have had hip or knee replacements; whether they required assistance when walking (use of a cane or walker); if they had difficulty hearing over background noise; and if they were afflicted with any vision impairments such as cataracts. People who affirmed to any of these impairments were excluded from participation in this study.
Individuals with PD were tested after a minimum 10 hour withdrawal of anti-Parkinsonian medications to satisfy the “Off” medication state; average withdrawal from medications was 14.7hours. Dopamine agonists are known to require longer time for their effects to subside because of their longer half-life compared to dopaminergic medications; consequently testing on these individuals occurred after a longer withdrawal period, once the half-life of the medication was surpassed. Whether individuals with PD were in their “Off” medication state was based on when they last took their anti-Parkinsonian medication; this was confirmed by assessment using the UPDRS (Table 1 for characteristics of individuals with PD). The healthy controls completed the study similarly to individuals with PD, however, they were not assessed using the UPDRS.
|
Participant |
Age |
Sex |
Time "Off" Medication |
UPDRS Score "Off" Medication |
Medication(s) |
|
PD1 |
56 |
M |
15 |
41 |
Requip, Deprenyl |
|
PD2 |
79 |
M |
10 |
29.5 |
Sinemet, APO-trihex |
|
PD3 |
64 |
M |
16 |
15 |
Sinemet CR |
|
PD4 |
73 |
M |
12 |
33 |
Simemet, Comtam |
|
PD5 |
74 |
F |
15 |
20 |
Lerocarb, Mirapex, Clonasepam, Comtam |
|
PD6 |
68 |
F |
11 |
26 |
Sinemet, Requip |
|
PD7 |
75 |
M |
12.5 |
49.5 |
Sinemet CR |
|
PD8 |
70 |
M |
13 |
23 |
Sinemet, Sinemet CR, Comtam |
|
PD9 |
50 |
M |
27.5 |
22.5 |
Sinemet |
|
PD10 |
54 |
F |
16 |
30.5 |
Sinemet, Mirapex, Clonazepam, Comtam |
|
PD11 |
70 |
F |
17 |
32.5 |
Sinemet, Mirapex, Amantidine |
|
PD12 |
70 |
M |
14 |
27.5 |
Sinemet CR, Comtam |
|
PD13 |
66 |
F |
10 |
13 |
Sinemet |
|
PD14 |
65 |
F |
13 |
29 |
Sinemet |
|
PD15 |
73 |
M |
13.5 |
No medication |
|
|
PD16 |
61 |
M |
15.5 |
No medication |
|
|
PD17 |
71 |
F |
14 |
34.5 |
Sinemet CR, Comtam |
|
PD18 |
77 |
M |
12.5 |
23 |
Sinemet |
|
PD19 |
64 |
M |
16 |
42 |
Sinemet, Mirapex |
|
PD20 |
71 |
F |
24 |
21.5 |
Sinemet |
Table 1 Individuals with PD participant characteristics inclusive of age, gender, sex, time “Off” anti-Parkinsonian medication, UPDRS scores and medications Note. The Unified Parkinson’s Disease Rating Scale (UPDRS) is presented as the total sum of the 31 criterion; each item was graded based on severity from 0 through 4).
Apparatus and Data Collection
All subjects walked barefoot beginning three meters before the 4.27meter computerized data-collecting and pressure sensitive carpet (GAITRite®, CIR System, Inc., Clifton, NJ, USA). They were instructed to continue walking three meters beyond the carpet; thus avoiding accelerations and decelerations associated with initiation and termination of gait. The carpet was located in a large, clutter-free laboratory that was darkened (by dimming the lights), though the ability to see limbs remained.
Participants were asked to wear a visual feedback device (Yoram Baram, Technion University, Israel) that was secured to a pair of non-prescription glasses. The visual feedback devise consisted of: a motion sensitive power source that was attached to the participant’s waistband, a mounting clip, and an adjustable arm to which a small screen was attached that projected into the eye. The adjustable arm was moved to accommodate the participant’s visual field, such that the white transverse lines were clearly seen when looking at the black screen. The device did not allow: the superimposition of the participant’s foot onto the projected moving parallel lines of the screen as he or she progressed forward; provide additional light to the surrounding environment, and it did not completely obstruct their normal vision. In addition, the mounting clip of the feedback device was not generic, and as such the screen could not be secured to any pair of glasses. Consequently, to optimize the perception of the visual stimulus, myopic and hyperopic participants were asked to try wearing their prescription glasses under the non-prescription glasses to which the screen was attached. If he or she felt that they lines in the field were clearer than without their glasses, they completed the experiment wearing both their own glasses and the non-prescription glasses.
Procedure
For all conditions whether the visual feedback device was providing optic flow or not, the glasses and power source were worn by the participants. This was to ensure that any changes in gait that may have occurred as a result of wearing a foreign device were accounted for equally in all conditions. Participants completed ten trials of each condition and were accompanied by a spotter as he/she walked. Three experimental conditions were carried out in a random order in the darkened room: i) Normal vision: subjects walked at a self-selected pace across the carpet, ii) Ground cues: participants walked at a self-selected pace while attempting to accurately contact the stripe locations on the ground with their heels, and iii) Optic flow cues: subjects walked across the carpet while wearing the visual feedback device. In the third condition, participants were instructed to try and step toward the progressing lines with their heels. The speed that the lines appeared to flow in the screen was equivalent to the pace he or she was walking because the device was sensitive to the frequency of the participant’s vibration. The third condition, of parallel stripes on the floor, was created using a black vinyl walking surface that was laid over and secured to the computerized carpet. White stripes, 2.54cm in width were spaced 65.5cm (normal adult step length12) apart along the length of the black overlay surface (4.27m). The contrast of the black surface with white lines was used for continuity; it mimicked the output from the screen of the visual feedback device.
Additionally, a fourth condition was administered last involving optic flow as the visual stimulus from the visual feedback devise while attending to an auditory metronome to maintain the pace. The frequency of the metronome was participant-specific depending based on their cadence data from the first condition (as determined by the computerized carpet). The cadences from the ten trials were averaged and the metronome was set to the closest frequency on the metronome. Since the metronome was capable of outputting in intervals of 4Hz average frequencies were rounded down to the closest frequency of output (Table 2). The subjects were asked to try to step towards the progressing lines with their heels while keeping pace to the metronome.
|
Individuals with Parkinson’s Disease |
Healthy Age-Matched Controls |
||||
|
Participant |
Frequency of Metronome (Hz) |
Average Frequency (Hz) |
Participant |
Frequency of Metronome (Hz) |
Average Frequency (Hz) |
|
PD1 |
108 |
103.5 |
HC1 |
88 |
108 |
|
PD2 |
108 |
HC2 |
120 |
||
|
PD3 |
108 |
HC3 |
104 |
||
|
PD4 |
100 |
HC4 |
104 |
||
|
PD5 |
80 |
HC5 |
108 |
||
|
PD6 |
108 |
HC6 |
108 |
||
|
PD7 |
94 |
HC7 |
120 |
||
|
PD8 |
96 |
HC8 |
116 |
||
|
PD9 |
100 |
HC9 |
112 |
||
|
PD10 |
104 |
HC10 |
100 |
||
|
PD11 |
108 |
HC11 |
120 |
||
|
PD12 |
96 |
||||
|
PD13 |
100 |
||||
|
PD14 |
108 |
||||
|
PD15 |
96 |
||||
|
PD16 |
100 |
||||
|
PD17 |
116 |
||||
|
PD18 |
108 |
||||
|
PD19 |
112 |
||||
|
PD20 |
120 |
||||
Table 2 Metronome frequencies of individuals with PD and healthy age-matched participants based on their average cadence during the normal walking condition
Custom software (GAITRite® GOLD CIR Systems, Inc., Clifton, NJ, USA) was used to determine the gait kinematics of the subjects for each of the trials. Data obtained from the individuals with PD were analyzed in a two-factor repeated measures analysis of variance (ANOVA). The “Control” and “Off” groups were the independent between- group variable with all other factors functioning as within-subject variables. Within-subject variables included: velocity, step length, step time, double support time and cadence. The resulting ANOVA was Time (PD OFF, Control) ∙ (Normal, Optic flow, Ground lines, Optic flow plus) ∙ (Trial 3, Trial 4…Trial 8). Trials one and two were disregarded in case of unfamiliarity of the participants with the device, and trials nine and ten were not counted to account for participant fatigue. Significant data with a p-level less than 0.05 were examined by Tukey’s HSD post hoc analyses.
Velocity
Analysis of gait across the four conditions revealed that individuals with PD walked slower compared to healthy age-matched control participants as demonstrated by a main effect for group F(1, 26)=24.82; p=0.000035 (Table 3). In addition, a main effect for condition demonstrated that only the optic flow condition negatively influenced speed of walking. F(3, 78)=24.15; p=0.000000. Post hoc analysis confirmed that velocity during the optic flow condition (when participants were required to focus attention on the illusion of progressive lines to step toward in attempt to change step amplitude) was the only condition to slow velocity. The interaction between group and condition was not significant, indicating that the velocity of individuals with PD and healthy participants was affected similarly by external cueing and that the velocity of individuals with PD did not improve with any of the external cueing strategies (confirmed by post-hoc).
|
PD |
Controls |
|
|
Velocity (cm/sec) |
91.7 |
120.26 |
|
Step length (cm) |
57.57 |
67.54 |
|
Step time (seconds) |
0.64 |
0.57 |
|
Double support time (seconds) |
0.34 |
0.23 |
|
Cadence (steps/min) |
95.41 |
103.91 |
Table 3 Mean characteristics of self-paced gait of individuals with PD and healthy age-matched control participants (* indicates difference between groups)
Step length
Within group analysis of step length revealed that individuals with PD walked with overall smaller step length than healthy participants F(1,26)=11.71; p=0.002069 and the main effect of visual cueing was significant F(3, 78)=5.15; p=0.002680 (Table 3). These effects were superceded by a two-way interaction between group and visual stimulus condition F(3, 78)=6.66; p=0.000461 (Figure 1). Post-hoc analysis confirmed that the step length of healthy participants did not significantly change across the four conditions, indicating no influence of visual cueing. Of course during the normal walking condition, the step length of individuals with PD was less than the step length of healthy participants. Optic flow failed to improve the step length of individuals with PD to that of healthy participants, however, the ground lines did improve the step length of individuals with PD. In fact, the ground lines condition was the only condition that elicited a normal step length in individuals with PD (compared to the healthy controls). In addition, the step lengths of individuals with PD during the optic flow plus condition did not differ from their normal step lengths and from their step lengths produced by the optic flow condition.
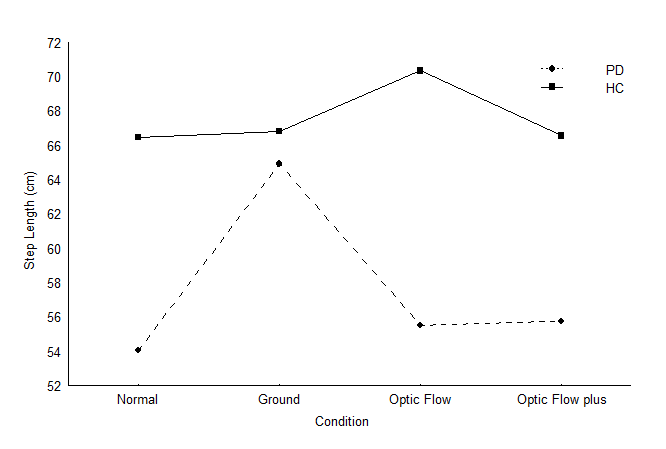
Figure 1 Step length of individuals with PD and healthy participants in the visual cueing conditions.
Step time
Analysis of gait revealed that PD had considerably longer time to complete a step than did the healthy-age matched counterparts F(1, 26)=5.74; p=0.024104 (Table 3). A significant main effect for condition was observed F(3, 78)=14.20; p=0.000000 indicating that participants had longer step times during the optic flow condition compared to the other conditions. The ground lines condition also yielded longer step times relative to both the normal walking and optic flow plus conditions (confirmed by post-hoc. As expected there was no significant difference between the step time of the normal walking condition and the optic flow plus condition, since the metronome forced participants to maintain the step frequency determined by their average cadence of normal walking. Therefore, the optic flow condition caused prolonged step time relative to the other conditions, but not any more than the ground lines condition (confirmed by post-hoc). F(3, 78)=2.78; p=0.046385 (Figure 2).
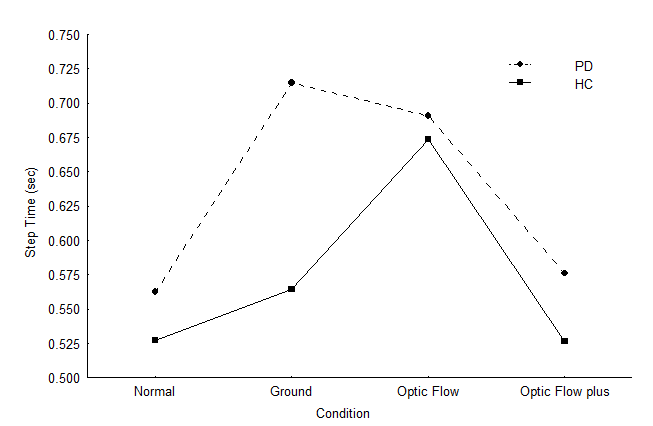
Figure 2 Step time of individuals with PD and healthy participants during the visual cueing conditions.
Double support time
A second temporal parameter, time spent in the double support phase, differed between the healthy participants and individuals with PD; double support time was significantly longer for PD than healthy participants F(1, 26)=5.39; p=0.028342 (Table 3). There were no interactions between group and condition illustrated by this timing measure, however, there was a significant main effect observed indicating that all subjects increased time spent in double support phase as a result of visual cueing condition F(3, 78)=4.06; p=0.009842. Post-hoc analysis confirmed that double support time during the optic flow condition was significantly greater than in the normal walking condition, ground lines condition, and optic flow plus condition.
Cadence
With respect to cadence, analysis revealed that both group F(1, 26)=10.08; p=0.003834 and condition F(3, 78)=35.73; p=0.000000 main effects were significant (Table 3). Beyond these main effects was an interaction between group and condition F(3, 78)=3.25; p=0.026282. Figure 3 below illustrates the effects of condition on the cadence of individuals with PD and healthy age-matched controls. Post-hoc analysis revealed that during normal walking, the cadence of individuals with PD was similar to healthy participants, with the exception of the optic flow condition. Furthermore, cadence of individuals with PD did not differ significantly between the optic flow condition and the ground lines condition. In the ground lines condition, individuals with PD had significantly lower cadence compared to the optic flow plus condition. Cadence of individuals with PD in the ground lines condition was significantly less than healthy participants in all conditions other than the optic flow condition. The optic flow condition yielded a lower cadence in both healthy and PD alike compared to the other conditions. Cadence for individuals with PD in the presence of optic flow was significantly lower compared to cadence produced by attending to the auditory metronome, to healthy participants in the normal walking condition, the ground lines condition, and the optic flow plus condition. In addition, the cadence of individuals with PD during the optic flow plus condition greatly differed from that of healthy participants during the same condition and during the optic flow condition. To summarize, though the ground lines decreased cadence in individuals with PD compared to their normal cadence and had insignificant effects on the cadence of healthy participants, optic flow significantly hindered cadence in PD and healthy alike compared to the other three conditions.
Summary
This study was unique because the experimental conditions were administered to participants such that their walking was genuine; they were not required to walk on an unusual apparatus such as a treadmill. Furthermore, we believe this study is the first to separate kinesthetic feedback from vision; optic flow was provided to the eye directly, rather than at the feet. The primary objective of this study was to determine how optic flow may be responsible for gait improvements of individuals with PD. In accordance with previous studies, the results of the current experiment confirm that normal stepping patterns can be elicited in individuals with PD when visual step cues are provided on the walking surface.1 Optic flow has been argued to be the underlying contributing factor of improving gait hypokinesia,10 though we believe previous research has not explored whether there are inherent mechanisms used by the brain in addition to the stimulus that allow the improvements to occur. The novel finding of this study is that step length cannot be increased solely by the provision of optic flow in individuals with PD; it may be that only when visuoproprioceptive feedback is available that step length of individuals with PD increase to that of their healthy counterparts.
Effect of visual cues on walking surface
In accordance with previous research,1,12 we found that providing visual step cues for participants increased step length of individuals with PD. Interestingly, it was also noted that visual cues on the walking surface lead to a significant decrease in cadence, and so there was no overall improvement to velocity. A more recent by Azulay et al.,10 demonstrated that improvements to both step length and velocity could be achieved when visuospatial cues were placed 45cm apart. In our study, participants were required to step on lines spaced 65cm apart, which was larger than their normal walking step length. It may be that the larger step length cue in the current experiment caused individuals with PD to pause in order to generate the required force to produce the larger step amplitude.
It is also important to note, that diminished cadence was identified for individuals with PD in both conditions where visual cues were provided. This increase relative to normal walking can perhaps be explained by a greater amount of time required to integrate the proprioceptive and visual information at a higher cortical level, since this condition removed the fundamental automaticity of walking, which is voluntary control of step length.13
For healthy age-matched controls, the effects of the visual step cues were not observed spatially or temporally. The step length required was not extraordinary for them and the task likely did not remove the automaticity of walking to the same extent as the optic flow condition.
In contrast to the visual feedback device, the transverse ground lines as visual stimulus allowed participants to visually confirm along with proprioception, that his/her foot matched the lines. Keisjers et al.,14 found that when individuals with PD had only proprioception available in pointing to a remembered target, their accuracy was markedly worse than healthy participants. However, when vision and proprioception were available, accuracy improved, leading to the conclusion that vision compensates for deficits in proprioceptive information.14 This is similar to the findings of many studies that have documented transverse ground lines as an effective means of increasing step length in individuals with PD;1,4,10 our results confirm this finding. There is much debate as to the cause of these improvements; recent research propose that it may be a result of a cerebellar pathway wherein visuoproprioceptive feedback bypasses the basal ganglia1,6,10 or as Schubert et al.,15 claim, there is a re-weighting of visual and proprioceptive feedback in the brain. Obviously more research is required to determine the underlying mechanisms that allow for improvements of gait, though we favour the former hypothesis that vision allows proprioception to bypass the damaged part of the brain where it can be properly integrated.
Effect of optic flow
Slower velocity observed in individuals with PD compared to healthy, age-matched controls has been attributed to smaller step amplitude rather than impaired timing control.1,4 The results of our study concur with this knowledge since step length of individuals with PD, not cadence was significantly less than healthy participants Schubert et al.,15 found that individuals with PD, who walked in front of a hemispherical screen mimicking optic flow, had increased velocity and stride length. They suggested that dynamic visual cues were responsible for both improvements to step length and velocity. It is important to note that in this experiment, the participants always had kinesthetic feedback available. In our study, we attempted to isolate the contributions of kinesthesia and optic flow by providing visual cues that were not produced from self-motion so that other feedback was limited. The results of our study demonstrate that when optic flow was the primary source of feedback available to guide walking, both individuals with PD and healthy participants decreased their velocity, while none of other conditions resulted in this decrement. Velocity impairments may be related to both step size and cadence (as indicated by double support time) deficits.1 and so it is important to consider the relative contributions of spatial and temporal characteristics to gait velocity.
As expected, individuals with PD walked with smaller step length than healthy control participants during normal gait, and this was not affected by the provision of optic flow. The faulty proprioceptive feedback available caused smaller steps relative to healthy participants because visual confirmation was not available to compensate for the diminished external cueing. Smaller movement amplitude was also noted by Desmurget et al.,16 when proprioception was primary source of feedback available to individuals with PD when pointing towards a target, indicating that vision of the limb is required to improve target accuracy.
Our results confirm previous studies which cite cadence as unaffected by PD.1,17 In the optic flow condition, decreased cadence was revealed for both individuals with PD and healthy participants alike. It may be argued that the optical flow device acted as a distracter causing individuals with PD to focus on their visual experience rather than gait. Perhaps cadence decreased more during this condition because individuals with PD took longer to judge the required step amplitude to match their foot to the lines in their visual field. The ground lines condition did not have the same affect on cadence because no estimation of step amplitude was required since participants were restricted to a set length. Increased double support time, may be a means of increasing proprioceptive feedback when vision is not available to compensate.6 More proprioceptive feedback may have been required to achieve balance because of disturbances. A feasible explanation for increased step time might be that longer preparation for the movement causes longer execution time.18
The intent of optic flow plus condition was to elucidate whether the benefits to step length from optic flow could be maintained while attenuating to a set pace. As a consequence of the ineffectiveness of the device to bring forth larger steps in individuals with PD, it is difficult to ascertain the implications of the metronome as a secondary task. It is well known that individuals with PD are able to maintain pace to an auditory metronome cueing at 85 to 115% of their normal cadence.19 In our study, individuals with PD and healthy participants were able to maintain pace with the metronome as it was set to their approximate normal walking pace. A study that expanded on the findings of Morris et al.,12 determined that when individuals with PD walked while carrying a tray of glasses, mimicking a dual task scenario, gait deteriorated.21 Conversely, when participants were asked specifically to attend to walking under dual task situations, there were no implications on gait performance.20 In our study, participants were also asked to focus on their walking while keeping pace to the metronome, so potentially there would have been no implications on gait had the device worked. In agreement with Freeland et al.,21 our study indicates decreased double support time when participants attended to the auditory cue; however, no increase in velocity was observed as a result of intervention with the metronome. In attending to the metronome, the deficits in cadence associated with the optic flow condition were ameliorated, but one might speculate that as previous studies have noted, individuals with PD tend to decrease movement amplitude to maintain pace.22 Had the device alleviated the gait-related symptoms, another potential outcome of attending to the metronome could have been favouring the auditory cue over the visual cues. This is plausible considering that walking is such an automatic process involving the spinal cord and brain stem, and that the most automatic motions are associated with movement amplitude.130 As a result, in attending to the secondary task (metronome), insufficiency in the automaticity of the movements may be heightened.23 The consequence of this outcome would be that even if the device were effective, it may be impractical because attention in its entirety would have to be on the visual stimulus, which would be very difficult to do.
Throughout the experimental conditions, there were no effects on the spatial parameters of gait in healthy participants. Since the basal ganglia are not damaged in healthy participants, this further supports that proprioception is properly integrated in the brain and that movement amplitude is executed normally. The decrease in velocity and increased step time and double support time observed only during the optic flow condition, indicates that normal rhythmicity of walking may have been removed in focusing attention on how they walked.
The visual feedback device used for this study provided optically flowing lines to the participant’s visual field, but did not allow the participant to visually confirm that his or her foot matched the lines. In other words visual feedback of self-motion, even from peripheral vision, was not available to the participants, in spite of the fact that the screen of the visual feedback minimally obscured the visual field of the right eye. The lack of visual feedback during this condition caused participants to be reliant on kinesthetic feedback, as a means of confirming body position and the completion of each step discretely. It is likely that a proprioceptive stimulus in healthy participants is a sufficient indicator to the brain that the action is complete, and that the next action in the sequence can begin.2,3 However, in PD, integration of proprioceptive information in terms of sensing body position may be impaired at the fault of damaged basal ganglia;6,14,17 consequently, proper execution of sequential movements and cueing of the next action in the sequence could not occur.2,24 Though proprioception was obviously available to individuals with PD, in the brain this type of feedback has few implications in providing the necessary cues to execute movement because it travels to the damaged basal ganglia. Instead, individuals with PD rely on internal cues that are generated by the motor system, not in response to an external stimulus, to complete the execution of the next action in a sequence.2,4,6 As such, the external cueing of this condition failed, causing increased reliance on internal cueing which is insufficiently produced by phasic activity in the cells of the globus pallidus.25,26 Morris and colleagues27 propose that diminished internal cueing could reduce movement amplitude through the sequence, although cadence has been shown to not be regulated by internal cueing.1,4 Our study has provided an important clue to the effectiveness of visual stimuli, suggesting that in order to improve gait of individuals with PD, vision and proprioception must be available. Though the mechanisms in the brain that allow vision along with proprioception to bypass the basal ganglia are not known, clearly the concurrent availability of vision and proprioception is required to elicit improvements in step length. Failure of the optic flow device to produce similar benefits to visual cues available on the walking surface may be linked to a lack of confirmation of self-motion. If proprioception is the only source of feedback to formulate a perception of self-motion, and this system is impaired in PD, then participants with PD would benefit from the optic flow device as much as they would from visual cues provided on the walking surface. This suggests that conscious perception of motion is necessary to herald improvements in locomotion and that the integration of vision and proprioception is an important part of this perceptual process. Future research should be directed toward the mechanisms in the brain that allow for improvements of gait.
This project was funded by the Biology Department. We would like to thank Dr. Yoram Baram for providing the device and Chad Lebold for their help with this study.
None.
None.

©2016 Almeida, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.