Journal of
eISSN: 2373-6410


Research Article Volume 4 Issue 5
1Laboratory of Neuropathology, First Department of Neurology, AHEPA Hospital, Aristotelian University of Thessaloniki, Greece
2Instiute of Alzheimer ?s disease Research, Heraklion Langada, Greece
3Laboratory of Forensic Medicine and Toxicology, Aristotelian University of Thessaloniki, Greece
Correspondence: Foivos E Petrides, Laboratory of Neuropathology, First Department of Neurology, AHEPA Hospital, Aristotelian University of Thessaloniki, Greece, Tel 302310993301
Received: February 27, 2016 | Published: April 25, 2016
Citation: Petrides FE, Mavroudis IA, Dados D, Manani MG, Chatzinikolaou FG, et al (2016) Dendritic Alterations of the Pyramidal Cell of Reil’s Insula in Alzheimer’s disease. J Neurol Stroke 4(5): 00154. DOI: 10.15406/jnsk.2016.04.00154
Alzheimer's disease is an insidiously progressive severe presenile and senile dementia, involving a number of cellular and biochemical mechanisms. It is characterized by cognitive decline affecting memory primarily, associated frequently with behavioral and mood disorders, which increasingly appear as the disease advances. Despite the strong clinical rationale and many laboratory hints, the insula may be an important target of Alzheimer's disease, neuropathologic alterations of Reil's insula have not been described extensively In the present study, we attempt to describe the morphometric and morphological changes of the neurons of insula in Alzheimer's disease in comparison to normal ageing, senile plaque deposition and neurofibrillary degeneration using the silver impregnation technique. Each one of the selected cells was traced using the Neuromantic application and the tracing was quantitatively analyzed with Image J program based on Sholl analysis method of concentric circles. Statistical analysis was based on the Student’s test on the basis of 360 cells in SPSS v.17.0 and significance was taken as p.
Keywords: Dendritic Alterations, Pyramidal Cell, Reil’s Insula, Alzheimer’s disease, Senile dementia, Sholl analysis method, Neurodegenerative disorder, Neurofibrillary tangles
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the brain, which appears in presenile and senile, affecting millions of people as the most common cause of memory decline around the world. AD is mainly characterized by the loss of or impairment of memory and other cognitive abilities in association with changes in mood and personality, autonomic disorders and disorientation as the disease progresses over time.1
According to recent research AD affects approximately 6-8% of people over the age of 65 years and increases in 30% in people older than 85 years.2,3 Neuropathologically, Alzheimer’s disease is mainly characterized by loss of neurons.4 marked synaptic alterations.5,6 morphological mitochondrial abnormalities.5-10 tau pathology.11 in the form of neurofibrillary tangles (NFT), inflammatory activation and mostly by deposition of Αβ polymers in the neuropil space as neuritic plaques.12-14 in the entorhinal cortex, hippocampus and many subcortical structures, which are involved in cognitive function.7,9
Accumulation of amyloid-β protein, which is the cleavage product of the amyloid precursor protein (APP).15 is considered to be as the pathologic hallmark for the neuropathological diagnosis of the disease. In addition, mitochondria, microtubules and Golgi apparatus are mostly pathologically involved in Alzheimer’s disease.8
Macroscopically, brains of AD individuals present cortical and subcortical atrophy, being obvious, primarily in the temporal lobes and secondary in the frontal pole and fronto-orbital area.10 Light microscopy reveals deposition of neuritic plaques and neurofibrillary tangles in the cortex of brain hemispheres, synaptic alterations in entorhinal cortex and hippocampus as well as in other brain areas such as acoustic and visual cortices, frontal cortex and cerebellum as the disease advances.10,16,17
It is found that Alzheimer’s disease is associated to reduced levels of acetylcholine, noradrenaline, serotonin, and corticotrophin-releasing factors, as well as to glutamate and N-acetyl aspartate decrease.18 The degeneration of cholinergic neurons in the basal-forebrain-cholinergic system that projects to the neocortex, hippocampus and other brain areas may play a substantial role in cognitive decline.18,19
The insular cortex plays important role in a variety of regulatory mechanisms ranging from visceral control and sensation to covert judgments regarding inner well-being.20-22 Focal pathology of the insular cortex caused by tumours, stroke, or gliosis often induces gastrointestinal, vasomotor, cardiovascular, and other visceral disturbances.23-26 The leading causes of death in Alzheimer disease include myocardial infarction, cardiac failure, and respiratory infections.27,28 All of these failures of visceral organs may be triggered or exacerbated by autonomic dysfunction.29-31 and insular pathology might play a crucial role.
Human insula is one of the paralimbic structures which is encased within the brain underneath the Sylvian fissure and is covered by the temporal, frontoparietal and fronto-orbital opercula. The insula is only visible after removing the operculae and has the shape of an inverted pyramid. Additionally insular cortex covers the claustrum and the basal ganglia.32
involved in Alzheimer disease. Neuroimaging studies have revealed atrophy of the insula.33-35 whereas other studies have shown changes in insular blood flow.36 biogenic amine concentrations .34 muscarinic receptor densities.37 metabolic activity.38 monoamine oxidase-B activity .34,39 and decreased RNA and protein content.40 Despite the strong clinical and many laboratory rationale hints that the insula may be a target of AD, neuropathological alterations of the insula in AD have not been described extensively.
In the present study, we tried to delineate the morphological and morphometric changes of pyramidal neurons of insula in AD in comparison to normal ageing, using silver impregnation technique.
Subjects
Tissue samples were obtained at autopsy from 8 neurologically normal individuals, and 7 from patients who suffered from Alzheimer’s disease, all of them aged 52-75 years.
All the cases were examined for their medical history and the appropriate information concerning their health condition was collected. The definite diagnosis of AD was based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA) criteria.41
Tissue selection and processing
All brains were immersed in 10% neutral buffered formaldehyde at least for 40 days prior to staining. A tissue block from the anterior part of insula (agranular portion), was excised. Insula is revealed after removing the frontoorbital, frontoparietal, and temporal opercula (Figure 1). Tissue block were coded and used for Golgi silver impregnation techniques. Processed tissue was serially sectioned at 120μm with a vibrotome such that the preparation was vertical to the cortical surface and perpendicular to the long axis of the short gyri.
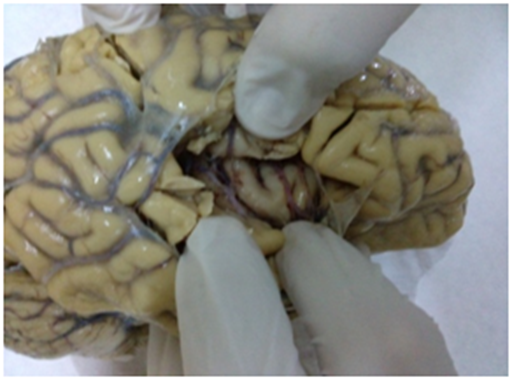
Figure 1 Reil insula visible while removing fronto-orbital, fronto-parietal, and temporal opercula cover.
Golgi method
According to protocol of silver impregnation technique, specimens were submerged in a solution of potassium dichromate (7g of potassium dichromate and 20ml of formaldehyde solution 37% in 300ml of distilled water) at room temperature in a photoprotective environment, where they remained for seven days. Then they immersed in a 1% silver nitrate solution, where they remained for seven days in a photoprotective environment at room temperature.
After the period of fixation, specimens were embedded in low-melting point paraffin and cut in thick sections at a range of 100-120μm. After gradual dehydration in alcohol solutions, specimens were mounted in Permount, between two cover slips and studied in Zeiss Axiolab Photomicroscope.
Cell selection criteria
Quantitative alterations for the examined neurons met the criteria set forth by Jacobs et al.42 that request uniform staining of neuronal dendrites, isolation from other impregnated cells and silver deposits (artifacts), good contrast between cells and background and relatively uniform tissue thickness.
Neuronal tracing and dendritic quantification
For each one of the 15 brains, 10 neurons from each area were selected. Every neuron was video recorded for 10-second at a magnification of 400x while the microscope table was moving at the standard velocity of 20μm/sec. Afterwards, the videos were analyzed in digital image sequences of 200 serial pictures, which were ultimately imported in Neuromantic application for 3D quantification of neuronal cells.
Each one of the selected cells was traced using the Neuromantic application. Neuromantic application is a freeware application programmed in Borland C++ Builder and it is a handy tool for neuronal 3D reconstruction. Its accuracy is comparable with the commercial application Neurolucida. Intuitive editing functionality combined with semi-automatic tracing and good visualization efficiencies, offers an alternative solution to commercial packages.43 Neuronal tracing started from cell soma and moved onto the basilar and apical dendrites.
Dendritic arbors were quantitatively evaluated in a centrifugal manner for apical and basal dendrites according to Uylings et al.44 Dendrites rising from the cell body are considered first-order segments, until the first symmetrical bifurcation. Dendritic branches arising from first-order segments are considered second-order segments, in turn, up to their symmetrical bifurcation into third-order segments, and so on. When asymmetric branching is met during the neuronal tracing, the offspring dendritic branch, recognized by a quantitatively thinner diameter, is classified as a next-order branch, whereas the parent dendrite would retain its order level past the branching point.
Dendritic measures and Sholl analysis
The parameters measured were (a) soma size, (b) total dendritic length, (c) cell contraction, (d) dendritic field asymmetry, (e) total number of dendritic segments and bifurcations, as well as (f) the length and number of dendritic segments per order. Image J program based on Sholl’s method for concentric circles used for quantitatively analysis.45 Concentric circles were drawn, starting from the center of the cell body, at intervals of 15μm, and counting the dendritic intersections within each circle.16 Staging of AD neurons made according to Braak and Braak classification.46 (Table 1).
Brain |
Age |
Gender |
Braak & Braak stage |
AD1 |
67 |
Male |
V/VI |
AD2 |
65 |
Female |
V/VI |
AD3 |
72 |
Female |
V/VI |
AD4 |
69 |
Male |
V/VI |
AD5 |
75 |
Male |
V/VI |
AD6 |
63 |
Female |
V/VI |
AD7 |
66 |
Male |
V/VI |
NC1 |
52 |
Female |
0 |
NC2 |
54 |
Female |
0 |
NC3 |
57 |
Male |
0 |
NC4 |
69 |
Male |
0 |
NC5 |
55 |
Male |
0 |
NC6 |
75 |
Female |
0 |
NC7 |
69 |
Male |
0 |
NC8 |
72 |
Female |
0 |
Table 1 Demographic features and Braak & Braak staging of the brains used in the present study
Statistical analysis
Statistical analysis was based on the Student’s test on the basis of 360 cells in SPSS v.17.0. Significance was taken as p<0.05. To ensure that autolysis time did not affect dendritic measurements, two-tailed Pearson product correlations were performed between all dependent measures and autolysis time.
Pyramidal dendrites from insula demonstrated severe reduction of total dendritic length, mainly correlated to loss of distal dendritic branches (Diagram 1A, 1B, 1D, Diagram 2D, Figures 1-5). Sholl’s concentric circles analysis revealed limitation of the dendritic field, while the number of intersections was significantly lower in distances greater than 169μm from cell soma for AD brains (Diagram 1C). Dendrite maximum for normal controls takes place at 160μm and with a critical value of 24, while in AD brains dendrite maximum were also at 160μm but the critical value was at 19,6.
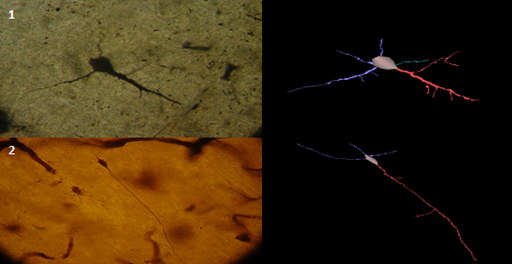
Figure 2 Pyramidal dendrites of the insula from AD individual, Golgi stain method, and its 3D reconstruction showing reduction of dendritic length, mainly correlated to loss of distal and dendritic branches (Neuromantic application). Scale bar=0,1μm.
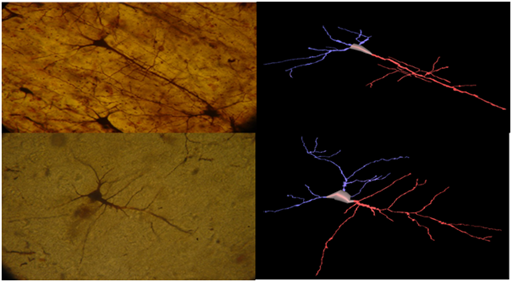
Figure 3&4 Pyramidal neurons of the insula from normal control brain, Golgi Silver staining method, and its 3D reconstruction (Neuromantic application). Scale bar=0,1μm.

Figure 5 Pyramidal neuron of the insula from normal control brain, Golgi Silver staining method, and its 3D reconstruction (Neuromantic application). Scale bar=0,1μm.
Besides dendritic loss, pyramidal cells from AD brains showed substantial changes regarding neuronal orientation and cytoskeletal integrity, while local and remote bifurcation tilts was significantly lower in AD brains (Diagram 2A). In addition, neuronal contraction was significantly different (Diagram 2B). Dendritic arbors of the pyramidal cells from NC were more symmetric than in AD brains (Diagram 2C).
Insula from AD brains demonstrated numerous senile plaques (Figures 6A, 6B), while Silver impregnation also revealed marked astrocytic proliferation (Figure 7).
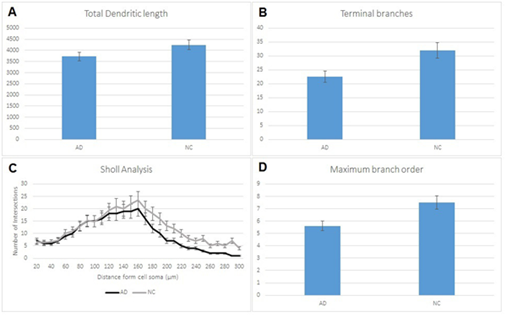
Diagram 1 (A) Reduction of total dendritic length in pyramidal cells in AD comparing to normal controls (NC) which is correlated to loss in terminal branches in AD (B). Sholl analysis of the dendritic field (C) showing lower intersections in distances greater than 169μm from cell soma in AD brains. (D) reduction in branches per order between AD individuals and normal controls (NC). Statistical analysis based on 360 cells. Significance was taken as p<0,05.
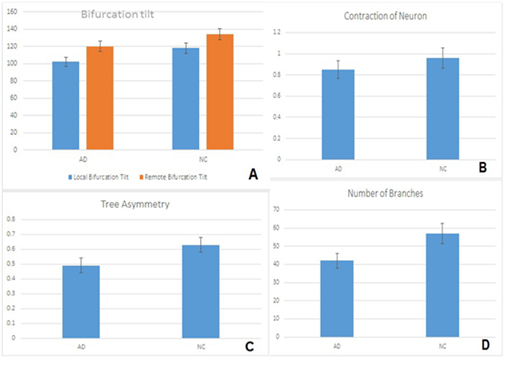
Diagram 2 (A) Changes in cytoskeletal orientation and integrity of pyramidal cells with reduction of local bifurcation tilt and remote bifurcation tilt between the two groups. Contraction of neuron in NC individuals comparing with AD individuals (B), differences on dendritic arbors of pyramidal cells in NC and AD individuals (C) and average number of branches in pyramidal cells (D). Statistical analysis of 360 pyramidal cells. Significance was taken as p<0,05.
In AD brains pyramidal neurons showed a significant loss of terminal branches. Dendritic branches are the most plastic components of the dendritic field.17 playing a crucial role in signal processing and cognition.47 The findings of the present study could be related to the neuroplasticity impairment that has been described in the insular cortex of patients suffered from schizophrenia.48,49 The loss of dendrites of pyramidal cells and interneurons leads to a substantial decrease of the synaptic contacts and a significant impairment of the pyramidal-interneuronal connectivity. Severe changes of dendritic orientation and dendritic bifurcation tilt were also observed. These changes are potentially related to the disruption of the cytoskeleton and its associated signal transduction proteins described by English et al.50,51 The aforementioned changes of dendritic polarity and dendritic field integrity could also be associated to abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in the described by numerous studies in Alzheimer’s disease.17,18,52
It is known that insula plays an important role in the sense of taste.53 which is impaired in Alzheimer’s disease.54,55 because insula as part of the primary cortical gustatory area contains neurons that may respond to gustatory stimuli.56,57 For this reason pathology of the insula may play an important role in recognizing and experiencing disgust.58,59 Although disgust in AD hasn’t studied extensively, however care givers of patients who suffer from Alzheimer’s disease witness that these patients often develop peculiar eating behaviours.60 The inability to recognize and experience food emotion may be an additional sensorial deficit of Alzheimer disease, which might be attributed to insula.61
In addition to impaired olfaction and gustation, which are involved in AD, other functions subserved by insula are also impaired. Insula unifies visceral sensory and motor functions and plays a major role in regulating autonomic responses.22,62,63 As AD is an irreversible progressive disease, patients with AD have a high mortality rate than age-matched controls.64 a fact which might be attributed to autonomic dysregulation and homeostatic disequilibrium.65
In Alzheimer’s disease insular pathology along with deficits in other brain structures and nuclei affects individuals in well-being, deregulate autonomic functions and interfere in social interactions and communication with other people.19,20
Multiple studies have suggested that the insula is pathologically involved in Alzheimer disease. Neuroimaging studies have revealed atrophy of the insula.32,34,66 whereas other studies have shown changes in insular blood flow.35 biogenic amine concentrations.33 muscarinic receptor densities.36 metabolic activity.37 monoamine oxidase-B activity.33,38 and decreased RNA and protein content.39
A study Bonthius et al.67 demonstrated that insula cortex is a protrusive target of Alzheimer’s disease and in many cases insula is heavily burdened with NFT’s and NP’s. These results show that behavioural function subserved by the insula is impaired in AD and insular dysfunction is part of Alzheimer’s symptomatology.
Olfaction is essentially impaired in many cases of AD.68-72 and sometimes dysosmia may be one of the primary phenomena of the disease. Thus some researchers suggest that olfactory tests may be applied in patients in order to discriminate AD from other brain disorders.55 Impairment of smell discrimination and identification reflects dysfunction at the cortical level and is considered that insula may be involved in olfactory dysregulation in AD.72
The morphological and morphometric estimation of pyramidal neurons from Reil’s insula in Alzheimer’s disease brains revealed a severe reduction of total dendritic length, mainly correlated to loss of distal dendritic branches, as well changes regarding neuronal orientation and cytoskeletal integrity. These changes lead to the essential decrease of the synaptic contacts and impairment of the pyramidal-interneuronal connectivity, as well as with other cortical and subcortical areas and may be related to impairment of regulatory mechanisms of insular cortex, ranging from visceral control and sensation to covert judgments regarding inner well-being.
Today, Golgi techniques remain virtually unique for demonstrating neurons and dendritic morphology in a biplanar or stereoscopic way, viewed by conventional light microscopy, equipped with a computerized image- analysis system. Golgi techniques contributed in broadening our views on neuronal development, maturation, plasticity, senescence and degenerating processes.73
None.
None.

©2016 Petrides, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.