Journal of
eISSN: 2377-4282


Research Article Volume 1 Issue 2
1Unit of Characterization and Structure of Materials, Zulian Institute for Technological Research - INZIT, Venezuela
2Laboratory of Materials and Emerging Technologies, Venezuelan Institute of Scientific Research-IVIC, Venezuela
3Laboratory of Fluids and Plasma, Venezuelan Institute for Scientific Research - IVIC, Venezuela
4Institute of Surface and Catalysis, University of Zulia, Venezuela
Correspondence: Jose Arevalo-Fester, Zulian Institute for Technological Research - INZIT, Km 15 La Canada de Urdaneta, Zulia State, Venezuela, Tel 58-4160164864
Received: October 26, 2014 | Published: November 22, 2014
Citation: Acevedo S, Arevalo-Fester J, Galicia L, Atencio R, Plaza E, et al. (2014) Efficiency Study of Silver Nanoparticles (AgNPs) Supported on Granular Activated Carbon against Escherichia coli. J Nanomed Res 1(2): 00009. DOI: 10.15406/jnmr.2014.01.00009
The antibacterial effects of Ag salts have been noticed since antiquity and currently are used to control bacterial growth in a variety of applications. Recent studies have revealed that the antimicrobial properties of silver are due to its ionized form, Ag+, and its ability to cause damage to cells by interacting with thiol-containing proteins and DNA. Silver nanoparticles are a form of silver of particular interest because of their easy production, high antimicrobial activity, and ability to be incorporated into a diverse range of products. We report the antibacterial properties of silver nanoparticles (AgNPs) supported on granular activated carbon (GAC). We prepare two types of GAC by using 60% (w/v) KOH and 85% (v/v) H3PO4 solutions respectively, as activating agent. AgNPs were synthesized by GAC impregnation with 0.1M, 0.05M and 0.01M AgNO3 solutions. Characterization was done using UV/V is spectrophotometry, Scanning Electron Microscopy (SEM) and adsorption isotherm data was used to determine the pore size distribution and surface area. Obtained materials showed nearly 100% antibacterial activities (elimination of microorganisms) against Escherichia coli because of the presence of the silver nanoparticles. Activity of the resulting material was related to the concentration of impregnated Ag and the surface area. Probably a high concentration of AgNPs on the GAC surface is more effective to attach to cell membrane and disrupt the permeability and respiration functions of the cell and thus kill the Escherichia coli.
Keywords:Silver nanoparticles, Escherichia coli, Activated carbon, Antimicrobial
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
Activated carbon (AC) is widely used in drinking water treatment to adsorb natural organic compounds, taste and odor compounds, and synthetic organic chemicals. Adsorbent property is because it is a highly porous material and provides a large surface area to which contaminants may adsorb. This is due to both the physical and chemical process of accumulating a substance at the interface between liquid and solids phases. Since AC filters will not remove microbial contaminants (such as bacteria and viruses), calcium and magnesium (hard water minerals), fluoride, nitrate, and many other compounds, combined methods are necessary to effectively treat the water. Previous studies have shown that antimicrobial formulations in the form of nanoparticles could be used as effective bactericidal materials.1-4 Indeed, nanosized inorganic particles, of either simple or composite nature, display unique physical and chemical properties and represent an increasingly important material in the development of novel nano devices which can be used in numerous physical, biological, biomedical, and pharmaceutical applications.5-7 A number of recent achievements offer the possibility of generating new types of nanostructure materials with designed surface and structural properties.7-10
It is well known that silver ions and silver-based compounds are highly toxic to microorganisms7-11 showing strong biocidal effects on as many as 16 species of bacteria including E. coli.12-15 Biocide mechanism is still under research. Some authors report that Ag inactivates microbes by interacting with their enzymes, proteins or DNA to restrain cell proliferation or cell division. It also binds to the negatively-charged bacterial cells to change the functionality of the cell membrane, thereby preventing bacterial regeneration.13,16-17 Thus, registration of biocidal silver and nanosilver products has increased dramatically over the last ten years, most likely as a result of improved capabilities in nanoscience and engineering that allow silver nanoparticles (AgNPs) to be formulated to confer increased durability and/or sustained antibacterial action, even under harsh environmental conditions.18 Based on these facts, we prepare granular activated carbon impregnated with silver nanoparticles (AgNPs-GAC), in order to determinate their antibacterial activity (elimination of microorganisms) against Escherichia coli.
Material
Phosphoric acid (H3PO4, 85% v/v) silver nitrate (AgNO3) and Tripticase Soy Agar (TSA) were purchased from Merck. Potassium hydroxide (KOH) and ammonium hydroxide (NH4OH) were obtained from Riedel de Haen and Fluka respectively. Eosin Ethylene Blue Agar Medium (EMB) was purchased from Himedia. Escherichia coli (E. coli) used in this research was ATTC 35218. For microscopic characterization a Scanning Electron Microscope (SEM), FEI Quanta 200 was employed. Surface area SBET was determined using a Micromeritics ASAP 2020 system. UV/Vis spectra were obtained by using a Biochrom Light wave II Spectrophotometer. Bituminous mineral coal was obtained from Carbonesdel Guasare (Venezuela).GAC preparation
GAC adsorbents were prepared according to literature method, starting from mineral coal. For GAC-1, concentrated (85% v/v) H3PO4, was used as activating agent, while GAC-2 was prepared using a (60% v/v) KOH solution. Pyrolysis was carried out for 2hours at 500°C and 1hour at 700°C, respectively.19
Nanoparticles impregnation
For GAC impregnation with silver nanoparticles, aqueous solutions (0.1M, 0.05M and 0.01M) of AgNO3 were prepared. In three 100 mL amber Erlenmeyers 1 g of GAC-1, was weighted and 20 mL solutions of AgNO3 at given concentrations were added in each Erlenmeyer, followed by 2 mL of concentrated NH4OH as Ag reducing agent (Tollens reaction). All samples were stirred for 24 hours and then filtered and washed with distilled water and finally dried at 120°C for 4hours. The same procedure was used for GAC-2.10,20-22
Electron microscopy characterization was performed and obtained images were analyzed in order to observe the presence of AgNPs on the GAC surface. Elemental analysis was done by using Energy Dispersive X-ray technique (EDX), included in the SEM configuration. Nitrogen adsorption isotherms were obtained at 77K and surface area SBET was determined by mean of Brunauer, Emmeret and Teller method.23
Bacteriological analysis
Efficiency of (AgNPs)GACs was analyzed using serial dilution technique in Petri dish. Tripticase Soy Agar (TSA) was used for inoculation of pure culture of Escherichia coli.
The minimum contact time was determined as follows: a 250 mL Erlenmeyer containing 100 mL of TSA was inoculated with pure culture of E. coli and incubated at 37°C for 18hours, until reaching a concentration of 108 CFU/mL, as determined by UV/Vis spectrophotometry at 600 nm wavelength. After that, 1gram of (AgNPs) GAC was added and kept under stirring for 60 min. These assays were performed in triplicate for each type of (AgNPs) GAC. Each 5 min, 1 mL of extract was taken and grown in EMB agar medium and incubated at 37°C for 24hours. CFU was calculated using equation 1
GAC characterization
Surface areas and pore volumes of the GAC materials activated with H3PO4 and KOH and their corresponding AgNPs are shown in (Table 1). The Tollen’s synthesis method gives silver nanoparticles with a controlled size in a one-step process.25 The basic Tollen’s method involves reaction 2, where Ag+ ions are reduced by aldehydes or carboxylates which are present on the GAC surface, in the presence of ammonia, yielding AgNPs.
GAC |
[Ag] |
SBET1 |
Vtpore |
Vmicro |
Vmeso |
Ag2 |
GAC-1a |
0.00 |
528.25±6.123 |
0.373 |
0.086 |
0.286 |
ND |
(AgNPs)GAC-1a |
0.01 |
423.22±8.717 |
0.214 |
0.125 |
0.089 |
7.49 |
(AgNPs)GAC-2a |
0.05 |
369.27±10.826 |
0.205 |
0.111 |
0.093 |
23.62 |
(AgNPs)GAC-3a |
0.10 |
309.04±6.573 |
0.155 |
0.093 |
0.061 |
29.56 |
GAC-2b |
0.00 |
1386.10±19.001 |
0.729 |
0.395 |
0.395 |
ND |
(AgNPs)GAC-4b |
0.01 |
698.62±15.777 |
0.364 |
0.238 |
0.125 |
8.86 |
(AgNPs)GAC-5b |
0.05 |
673.74±15.142 |
0378 |
0.225 |
0.143 |
27.16 |
(AgNPs)GAC-6b |
0.10 |
615.16±16.048 |
0.305 |
0.240 |
0.064 |
42.37 |
Table 1 Surface areas and pore volumes
*SBET1 : Values are means ± SD of triplicate determinations; Ag2: Elemental analysis was determined by Energy-Dispersive X-ray Spectroscopy (EDX); aActivated with H3PO4; bActivated with KOH, ND: Not Detected Minimum contact time test
Table 1, shows a relationship between Ag concentration in the impregnation solution and surface area SBET. The BET surface area diminishes when increasing the Ag content in the impregnation solution until the maximum saturation level is reached. A decreasing of pore volume is also observed due to Ag dispersion along all pore range without any specific size limitation.25-27
SEM characterization
SEM micrographs presented in (Figures 1 & 2) corroborate that the AgNPs content supported on the carbon matrix is related with the concentration of the impregnation solution and the surface area of the GACs. This fact was also corroborated by elemental analysis as shown in Table 1. Since nature and concentration of the reductant must play a major role in controlling the silver nanoparticle size, it was assumed that the higher surface area determined in GACs activated with KOH (higher concentration of reductant groups) favors the primary formation of a large number of small clusters with a low coagulation barrier; their further irreversible aggregation was responsible for the presence of coarser agglomerates as observed in (Figures 1A-1D & 2A-2D).28-29
Antibacterial efficacy results
Antibacterial inhibition test is shown in Figure 3. GACs used in this study were found to have various degrees of antimicrobial effects against microorganisms tested.GAC activated with H3PO4 and KOH (GAC-1 (Figure 3A-3D) and GAC-2 (Figure 3E-3H), respectively) were not effectives as antibacterial material as is also observed in Figure 4 & 5. These results also demonstrated that these materials were not capable to reduce significantly E. coli, without aid of AgNPs.
In Figure 4, E. coli concentration decrease when Ag nanoparticles in GAC are increased. For (AgNPs)GAC-3 (%Ag: 29.56 w/w), total E. coli was eliminated after 25 min of stirring. The same behavior was observed for (AgNPs) GAC-2 (%Ag: 23.62 w/w), while the GAC with the less quantity of Ag nanoparticles, (AgNPs) GAC-1 (%Ag: 7.49 w/w) was able to reduce E. coli after 45 min from t0: 16x107 UFC/mL to 96x105 UFC/mL After that, bacterial concentration remains constant until the end of the assay (60 min).
All (AgNPs) GACs prepared from GAC activated with KOH were in general, more time-efficient to eliminate E. coli, as shown in (Figure 5). For (AgNPs)GAC-6 (%Ag: 42.37 w/w), E. coli concentration decreased dramatically after 10 min with a final minimum contact time of 15 min. The same behavior was observed for (AgNPs)GAC-5 (%Ag: 27.16 w/w),with a minimum contact time of25 min. Finally, after 30 min of stirring, (AgNPs)GAC-4 (%Ag: 8.86 w/w), was able to eliminate E. coli.
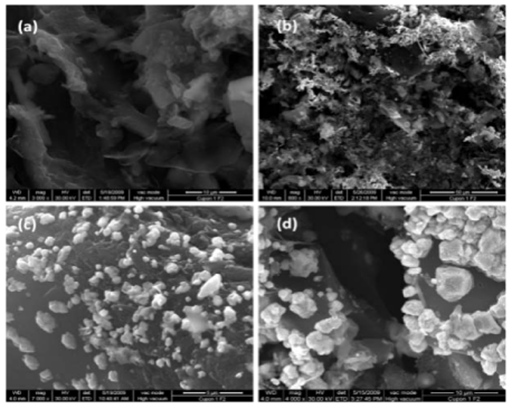
Figure 1 SEM micrographs of H3PO4 activated GACs.
a. GAC-1
b. (AgNPs) GAC-1
c. (AgNPs) GAC-2
d. (AgNPs) GAC-3
.

Figure 2 SEM micrographs of KOH activated GACs.
a. GAC-2
b. (AgNPs) GAC-4
c. (AgNPs) GAC-5
d. (AgNPs) GAC-6
.
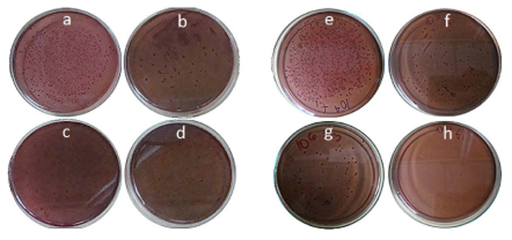
Figure 3 Antibacterial inhibition test of GACs and AgNPs GACs against E. Coli
a. GAC-0
b. AgNPsGAC-1
c. AgNPsGAC-2
d. AgNPsGAC-3
.
The results of this study showed that (AgNPs) GACs were able to eliminate E. coli. The elimination rate depends on the quantity of Ag adsorbed on GAC.3 Obviously, the adsorption phenomenon is related to surface area of the material. In this sense, GAC activated with KOH and impregnated with 0.1M AgNO3 solution, were more effective material for E. coli elimination, under employed experimental conditions. This results are in agreement with the above-mentioned antimicrobial properties of Ag, which is believed that the interaction between positively charged Ag ions and negatively charged bacterial membranes plays a major role in causing the membrane rupture and cell lysis. Researchers also reported that Ag+ prevents DNA replication or involves its interaction with thiol groups of proteins, blocking and inactivating of respiratory enzymes.30-32
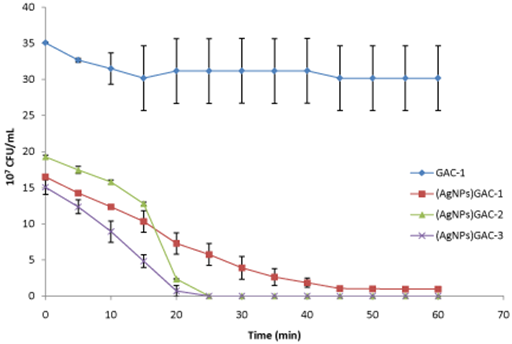
Figure 4 Activity of GAC activated with H3PO4 and their (AgNPs) GACs at different Ag concentrations against E. Coli. Ag content in %(w/w): GAC-1: 0; (AgNPs)GAC-1: 7.49; (AgNPs)GAC-2: 23.62; (AgNPs)GAC-3: 29.56 Values are the average of three individual replicates and error bars represent standard deviation .
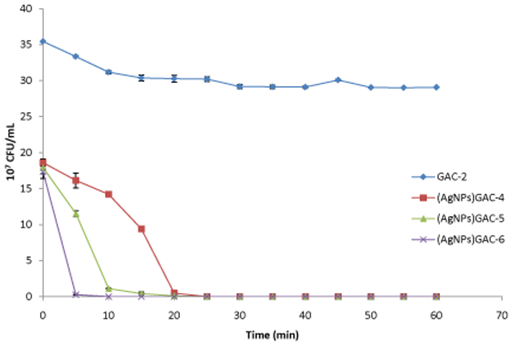
Figure 5 Activity of GAC activated with KOH and their (AgNPs) GACs at different Ag concentrations against E. Coli. Ag content in %(w/w): GAC-2: 0; (AgNPs)GAC-4: 8.86; (AgNPs)GAC-5: 27.16; (AgNPs)GAC-6: 42.37 Values are the average of three individual replicates and error bars represent standard deviation. .
The fact that the number of researches devoted to antibacterial and antiviral activity of AgNPs increases every year confirms the high interest in both synthesis and properties of silver nanoparticles. In this research we found that antimicrobial activity might be dependent on the surface area of GACs, AgNPs concentration and nanoparticles size as well as bacterial colony forming units (CFU). The obtained results from the (AgNPs) GACs assays demonstrate that they were effective as antibacterial against E. coli over a short period of time. In this sense, GAC activated with KOH and impregnated with 0.1M AgNO3 solution AgNPsGAC-6showed the higher AgNPs content (42.37 wt %). For this reason, it was able kill completely E. coli strain (from 107 CFU/mL to 0 CFU/mL) after 15 min of stirring. Nevertheless, in this work, experimental conditions employed are rarely found in real systems, it is possible to predict based on physical, chemical and biological properties of both GACs and AgNPs, that they could be combined to produce nanotechnology based devices for practical applications such as water treatment and recycling.
We acknowledge the financial support of FONACIT. (Project: 201100139).
None.

©2014 Acevedo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.