Journal of
eISSN: 2376-0060


Research Article Volume 4 Issue 1
Parul Instititue of Pharmacy, India
Correspondence: Asha Patel, Parul Institute of Pharmacy, Post Limda, Vadodara, Waghodia, Tel 9974727956
Received: January 18, 2017 | Published: March 7, 2017
Citation: Patel A. Exploring polymeric nano-particles as targeted pulmonary delivery of rifampicin, ethambutol and ofloxacin against inh-resistant tuberculosis. J Lung Plume Respir Res. 2017;4(1):23–35. DOI: 10.15406/jlprr.2017.04.00116
Present research focus on novel combination of Rifampicin, Ofloxacin and Ethambutol loaded polymeric nanoparticles for the effective eradication of isoniazid resistant species of Tuberculosis. The nanoparticles containing Rifampicin, Ethambutol and Ofloxacin were prepared by spray drying technique using biodegradable polymer PLGA through critical process as well as polymer attributes which were identified and screened using Plackett-Burman screening design. Partial least square equation generated using Minitab Software for processing parameters as flow rate- 5ml/min, inlet temperature- 60˚C, aspirator capacity- 50, ultrasonication time 40 min, ultrasonic amplitude % 60, and product parameters like drug: polymer ratio 2:1, aerosil concentration 1%, Solvent (DCM: Ethanol) ratio 75:25 on critical responses i.e. particles size, encapsulation efficiency, and product yield. Diffusion study revealed that optimum formulation containing 2:1 drug to polymer ratio was able to sustain the release of drugs up to 12hrs. Particles Size was found to be less than 100 nm with uniform spherical shape and good agreement using TEM analysis. The three drug combination showed significantly synergism (p 0.007) for isoniazid susceptible species during in-vitro antimicrobial assay prove better efficacy. Overall study explored potential use of polymeric particles as targeted delivery system for drug susceptible and isoniazid resistant tuberculosis treatment.
Keywords: polymeric particles, ofloxacin, rifampicin, ethambutol, PLGA
PB Design, plackett-burman screening design; PBF, plackett-burman formulations; Qbd, quality by design; OFX, ofloxacin, RIF, rifampicin; EMB, ethambutol; Pms, polymeric particles; RES, reticuloendothelial system; Doe, design of experiments; Cqas, critical quality attributes; %DC, percent drug content; %EE, percent entrapment efficiency; ANOVA, analysis of variance; TEM, transmission electron microscopy; FICI, fractional inhibitory concentration index; MIC, minimu inhibitory concentration
Recently, nano polymeric particles (PNs) have drawn major attention in drug delivery due to its potential features such as smaller particle size, good thermodynamic stability, increase in solubility of hydrophobic drugs and prolong drug release and avoid recognition by the reticuloendothelial system (RES).1–3 The polymeric nanoparticles comprise a drug-loading core and a hydrophilic shell. Amphiphilic block copolymer forms particles when in contact with an aqueous vehicle by self assembly resulting in hydrophobic interactions wherein hydrophobic drugs can be encapsulated into the central core of particles through hydrophobic interactions.4,5 PLGA is a promising excipient due to non- toxicity, biocompatibility, biodegradability, ability to enhance the penetration of large molecules across mucosal surfaces and bioadhesion properties. Polymeric particles are block copolymers self-assemble into spherical particles in water. A polymeric micelle usually consists of several hundred block copolymers and has a diameter of about 20-50nm. There are two spherical concentric regions of a polymeric micelle containing densely packed core consisting of hydrophobic blocks and a shell consisting of a dense brush of polymer. PLGA (Poly D,L lactide-co Glycolic acid) is a promising excipient that can be employed as biodegradable polymer due to non-toxicity, biocompatibility, biodegradability, ability to target the specific site for targeted drug delivery and it has gained attention for the formulation of spray dried PMs for pulmonary drug delivery.
Tuberculosis (TB) is a potentially fatal contagious disease caused by Mycobacterium tuberculosis, which most commonly affects the lungs. Tuberculosis can be treated by first line drugs (Isoniazid, rifampicin, Ethambutol, Pyrazinamide and Streptomycin); second line drugs (Flouroquinolnes, Aminoglycosides, Polypeptides, Cycolserine, Terizidone etc.) and third line drugs (Rifabutin, Thioridazine, Thioacetazone, Linezolid, Vitamin D etc.). In clinical trials, it was found that fluoroquinolones may also be useful in the treatment of drug-susceptible Mycobacterium tuberculosis in order to shorten the treatment. Rifampicin specifically inhibits bacterial RNA polymerase, the enzyme responsible for DNA transcription, by forming a stable drug-enzyme complex. Ethambutol interferes with cell wall biosynthesis in mycobacterium tuberculosis by inhibiting action of arabinosyl transferase. Ofloxacin inhibits action of DNA gyrase hence interfering with DNA supercoiling in mycobacterium tuberculosis. Treatment for TB using the standard oral antibiotic regimen requires high drug dosing and lengthy treatment times. Oral administration of high systemic doses of single or combined antibiotics causes unwanted side-effects by high systemic exposure and the long treatment times (up to 6months) treat the slow-growing populations of bacteria but also decrease patient adherence. Premature self-termination of treatment by patients in turn leads to drug resistant strains of TB which further complicates treatment efficacy.
Inhaled antibiotic particles can target alveolar macrophages which, when infected with TB, are maintained in a state of ‘alternative activation’ whereby macrophage cytosol conditions are suitable for TB bacilli replication and survival. However, phagocytosis of particles has been shown to revert these macrophages to a ‘classical activation’ state that resurrects their innate bacterial clearance mechanisms. Design of Experiment (DoE) provides a mean to determine the multi-factorial relationship among the input parameters that influence experimental output.6,7 Plackett-Burman (PB), a statistical screening design has been used to study the main effect on formulation.8,9 A Plackett-Burman design was employed to screen various factors on %yield, entrapment efficiency (%EE), and particle size for production of Ofloxacin, Rifampicin and Ethambutol loaded PMs to improve efficacy in treatment of tuberculosis.
Materials
Rifampicin was obtained as a gift sample from Sun pharma (Ankleshwar, India). Ofloxacin was obtained as a gift sample from Elite Pharma pvt. Ltd., India and Ethambutol was obtained as a gift sample from Macleods pharmaceuticals (Ankleshwar, India) Chitosan was a gift sample from Panvo Chemicals Ltd. (Chennai, India). Hydroxypropyl Cellulose was a gift sample from Elite Pharmapvt. Ltd., India. Sodium Hydroxide and Potassium Hydrogen Orthophosphate was a gift sample from Atul Chemicals (Anand, India). Dichloromethane and Ethanol was a gift sample from Astron laboratory (Ahmedabad, India). PLGA and Colloidal silicon dioxide was a gift sample from Evonik Industries (Mumbai, India). Methanol and Acetic acid was a gift sample from Astron laboratory (Ahmedabad, India).
Methods
Screening of polymers and solvents: Polymers such as PLGA, chitosan and Hydroxypropyl cellulose were chosen as biodegradable, biocompatible and have minimal toxicity. Solvents such as ethanol, methanol, dichloromethane and their combination were chosen on the basis of solubility of drug as well as polymer. For screening of polymers and solvents, the spray drying conditions were kept as inlet temperature- 50˚C, aspirator capacity- 45 and flow rate-5ml/min. Critical attributes % yield; moisture content and particle size were measured.
Drug excipient compatibility
Fourier transform infrared spectroscopy (FTIR): The Infra-Red spectra of Ethambutol, Rifampicin, Ofloxacin and polymers and physical mixture (Ethambutol, Rifampicin, Ofloxacin and PLGA) were obtained using Fourier Transform Infrared Spectrophotometer (Grams, Buck scientific Model-500) in order to detect the existence of interaction between drugs and polymers by dispersing a sample in KBr to prepare 10% of mixture and was grounded generally in mortar-pestle with KBr before being compressed into pellets. This pellet was placed in light path and spectrum was recorded at a resolution of 2cm-1 over a frequency range of 4000 to 400cm-1. The background spectrum of KBr was used as blank for determination.
Differential scanning calorimetric (DSC): Differential scanning calorimeter (DSC) was performed using DSC-60 Thermal analyzer, Shimadzu, Asia pacific, Japan to study the thermal behavior of Ethambutol, Rifampicin, Ofloxacin and polymers. The instrument comprised of calorimeter (DSC-60), Flow Controller (FCL-60), Thermal Analyzer (TA-60) and operating software (TA-60). The samples (2-4mg) were heated in hermetically sealed flat-bottomed aluminium pans under nitrogen flow (20ml/min) at a scanning rate of 10ºC/min from 25ºC to 200ºC. Empty aluminium pan was used as the reference standard.
Formulation of drug loaded polymeric nano-particles: Solvent evaporation method: Drug polymeric dispersion were mixed together and refluxed for 24h at 60ºC under constant stirring, then solvent is evaporated by flash evaporator under vaccum and sonic ate the final dispersion using ultrasonic homogenizer for 40 min. After sonication the resulting dispersion place in to the round bottom flask and it was run into the rotary vaccum evaporator with temperature 60ºC and 100RPM.
Spray drying process: Spray drying is a complex process and there are many factors like seven process parameters as mentioned below- 1. Aspirator, 2. The humidity of the drying gas, 3. Inlet temperature, 4. Spray gas flow speed, 5. Feed rate, 6. Concentration of drug, aerosil and polymer, 7. Solvent which can affect the quality of the product Drugs, polymer and aerosil were dispersed in DCM: Ethanol (75:25) solvent mixture. Sonic ate the final dispersion using ultrasonic homogenizer for 40min. After sonication, the resulting dispersion run into the spray drier with aspirator capacity 45, feed rate 5ml/min and inlet temperature 60ºC. Then collect particles from cyclone separator.
Development of drug loaded polymeric nano-particles by Plackett Burman screening design: Plackett-Burman Design was used for the influence of spray drying process parameters and product parameters on critical attributes of particles. A set of experiments, total of 11 experiments were performed for eight factors at two levels each using the PB screening design was adopted to prepare drug-loaded nano PNs. This design investigates every input factor and arranges them on the Pareto chart based on the magnitude of its influence with positive or negative sign respectively (color and colorless).10 PB design screens large number of input factors and at the same time reduces the number of runs.11,12 ‘t’ statistic is determined by estimating the standard effect of each input factor. The experimental runs with independent variables with their levels as per PBD shown in Table 1 and the observed responses like product yield, particle size and percentage entrapment efficiency are shown in Table 2. The factors with bar extending beyond the vertical line on the Pareto chart shows significant influence on responses at 95% confidence level.13 The factors show positive or negative sign on the Pareto chart reflecting increased or decreased effect respectively when moving from lowest to the highest level for the specific factor. Total twelve experimental trials involving eight independent variables were generated using. Minitab software shown in Table 3 selected independent variables and the product yield, entrapment efficiency and micelle size were set as response variables. The variables were correlated using the following polynomial equation with PB design.
Independent Variables |
Levels |
|||
|---|---|---|---|---|
Coded Value |
Transform Value |
|||
low |
high |
low |
high |
|
Drug: Polymer Ratio |
-1 |
1 |
1:01 |
2:01 |
Ultrasonication Time(min) |
-1 |
1 |
20 |
40 |
Ultrasonic Amplitude% |
-1 |
1 |
60 |
80 |
Aerosil Concentration% |
-1 |
1 |
0.5 |
1 |
Solvent (DCM: ethanol) ratio |
-1 |
1 |
50:50:00 |
75:25:00 |
Aspirator Capacity |
-1 |
1 |
45 |
50 |
Feed Rate(ml/min) |
-1 |
1 |
5 |
10 |
Inlet Temperature(0C) |
-1 |
1 |
50 |
60 |
Table 1 The experimental variables and level of PB design
Dependent Variables and their Desired Range |
|
% Yield |
65%-75% |
% Entrapment Efficiency |
80%-90% |
Particle Size |
less than 5 μm |
Table 2 Dependent variable and its desired ranges
Ind. Variables |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
Drug: Polymer ratio |
2:01 |
2:01 |
2:01 |
1:01 |
2:01 |
1:01 |
1:01 |
1:01 |
2:01 |
2:01 |
1:01 |
1:01 |
Ultrasonication time(min) |
40 |
20 |
20 |
40 |
20 |
40 |
40 |
20 |
20 |
40 |
20 |
40 |
Ultrasonic amplitude% |
80 |
60 |
80 |
80 |
60 |
60 |
80 |
80 |
80 |
60 |
60 |
60 |
Aerosil Concentration% |
0.5 |
1 |
0.5 |
0.5 |
0.5 |
0.5 |
1 |
1 |
1 |
1 |
0.5 |
1 |
Solvent (DCM: ethanol) ratio |
75:25:00 |
75:25:00 |
50:50:00 |
75:25:00 |
75:25:00 |
50:50:00 |
50:50:00 |
75:25:00 |
50:50:00 |
75:25:00 |
50:50:00 |
50:50:00 |
Aspirator capacity |
50 |
50 |
45 |
45 |
50 |
50 |
50 |
45 |
50 |
45 |
45 |
45 |
Feed rate(ml/min) |
5 |
5 |
10 |
5 |
10 |
10 |
10 |
10 |
5 |
10 |
5 |
5 |
Inlet Temperature(0C) |
60 |
60 |
60 |
50 |
50 |
60 |
50 |
60 |
50 |
50 |
50 |
60 |
Table 3 Design matrix generated from Plackett-Burman design
Y = A0 + A1X1 + A2X2 + A3X3 + A4X4 +…………+AnXn ……………………….Eq. 1
Where, Y is the response, A0 is the constant, and A1 is the coefficients of the response.11
Characterization of drugs loaded polymeric nano-particles.
Determination of particle size: Particle size was determined using laser diffraction technique (Malvern 2000 SM, Instruments, UK). The particle size measurements were carried out at a 90° scattering angle. The samples were dispersed in distilled water. The average particle size was determined and expressed in terms of d(0.9)nm.
Zeta potential analysis: The zeta potential was measured using the laser Doppler electrophoresis mobility measurement technique (Zeta Potential Measurement ZS 90, Malvern Instruments, UK) at a temperature of 25°C.
Transmission electron microscopy (TEM): The morphology of drugs loaded nano PMs was performed using transmission electron microscopy (Tecnai G2 Ultra twin FEI, Netherland). A drop of the sample was placed onto a carbon coated grid to form a thin liquid film. The excess solution was removed and sample was examined and photographed at an accelerating voltage of 120KV.
Critical micelle concentration:14 To verify and characterize the formation of particles, the CMC was determined by plotting the solubility of Sudan III stain (at an absorbance of 519nm) in aqueous solutions of the polymer derivatives against polymer concentration and observing the threshold above which particles from using Spectroflorimeter.
Evaluation of polymeric nano-particles
Entrapment efficiency:15 10mg of accurately weighed drug-loaded polymeric nanoparticles were added to 100 ml of methanol. The resulting mixture was shaking for 24hours on an orbital shaker incubator (Remi, RIS-24BL, and India). After suitable dilution with phosphate buffer (pH 7) analyzed by the developed and validated method of Zero order derivative spectroscopy using U.V spectrophotometer. (Data were not shown)
Encapsulation efficiency (%) was calculated by using following equation,
% Entrapment efficiency=(Practical Drug content)/(Theoritical Drug Content)*100……… Eq.2
In-vitro drug release study: The in vitro drug release test was performed with the help of Franz diffusion cell using Dialysis membrane 110. The receptor compartment was filled with 22.7ml Phosphate buffer pH 7.4 and maintained at 370C±0.50C. Samples were periodically withdrawn from the receptor compartment & replaced with the same amount of fresh buffer solution, and were assayed by UV spectrophotometrically at ZCP of each drug. Mechanism of drug release was determined using various kinetic models.
Aerodynamic properties evaluation: The aerodynamic properties of the powder were investigated using cascade impactor.16 20mg of each sample loaded into a hard gelatin capsule manually. The experiment was carried out at an air flow rate of 60 l/min. A capsule filled with particles was loaded and an actuation time of 4 s was allowed for each capsule to completely disperse all the particles. Particles remaining in the capsule, inhaler, throat, pre-separator, individual impaction plates, and stages were extracted using phosphate buffer. Amount of drug deposited on capsule, inhaler, throat, pre-separator, individual impaction plates, and stages was assayed using UV spectrophotometer at ZCPs. MMAD, GSD and % Emission was calculated.
In-vitro antimycobacterial study: Twelve INH-resistant clinical isolates (10 clinical isolates and the H37Rv reference strain) were studied.17 The MIC in combination with three drugs was studied by crossing five concentrations for each drug: the individual MIC found, one MIC above and three. In order to avoid seeding this number of plates simultaneously, an alternative methodology was developed in two consecutive steps. First, three concentrations of each drug were tested, including the MIC and the immediately lower and upper concentrations. In the second step, the three concentrations lower than the MIC was included. The Fractional Inhibitory Concentration Index (FICI) was calculated.18
Preliminary Screening of Polymer and Solvents
Based on preliminary screening, alone Dichloromethane (DCM) and ethanol, polymer gets precipitated out while in case of solvent blend of DCM: Ethanol (75:25), less particle size and more yield were obtained and in case of DCM: Ethanol (50:50),moderate yield and high moister content was seen in product as mentioned in the Table 4.
Solvents |
Parameters |
|||||
|---|---|---|---|---|---|---|
%Yield |
Particle Size(Μm) |
Moisture Content |
||||
PLGA |
HPC |
PLGA |
HPC |
PLGA |
HPC |
|
Methanol |
39 |
29 |
13 |
20 |
High |
High |
Dcm |
Precipitation of Ethambutol |
|||||
Ethanol |
Insolubility of Drugs |
|||||
Dcm: Ethanol (50: 50) |
43 |
33 |
9 |
18 |
High |
High |
Dcm: Ethanol (75: 25) |
62 |
40 |
5 |
18 |
Low |
Low |
Table 4 Preliminary screening of polymer and solvent
FTIR study
The FTIR spectrum indicated that various functional groups showed wavelength which denoted the structure of RIF, EMB and OFX shown in Figure 1. The results of IR spectra indicated absence of any well-defined interaction between Drug (RIF, EMB and OFX), polymer (PLGA).
Differential scanning calorimetry
The DSC patterns are represented in Figure 2. There was a negligible change in the melting endotherms and exotherms of the prepared physical mixture of Drug, Polymer and Excipient. Melting temperature of physical mixture was found to be almost same but with a slight reduction which is not significant. Sharp peaks were observed at 272ºC, 203ºC, 264ºC, and 76.67ºC temperature in DSC thermo grams of RIF, EMB, OFX and PLGA respectively concluded all the three drugs are compatible with each other and also with all the other excipients.
Screening design
PB design was applied as a screening method for identifying the most influencing significant factors.19 Prediction of the main effect of formulation and process parameters on the responses is a crucial requirement in the development of Drug-loaded polymeric nanoparticles by spray drying technique. Eight factors that may affect the experimental responses were selected as independent variables at two levels for the study which influences on responses were outlined in Table 5. Polynomial equations for individual response reflect the relationship between dependent and independent factors. The ANOVA results showed none effects have p-values less than 0.05, which indicates that factors are insignificantly different from zero at the 95.0% confidence level Table 6. The regression coefficient of particle size, % entrapment efficiency and % yield indicates 67.49 % of variability around the mean. The correlations between the factors on the response are as shown in the polynomial equation respectively.
Batches |
Design Matrix |
Response 1 (%yield) |
Response 2(particle size ) |
Response 3(%EE) |
1 |
+ + + - + + - + |
47.94 |
710 |
72.77 |
2 |
- - - + + + - + |
69.75 |
130 |
86.15 |
3 |
+ + - + + - + - |
55.12 |
1210 |
77.56 |
4 |
- + - - - + + + |
50.67 |
730 |
71.23 |
5 |
+ - - - + + + - |
51.23 |
870 |
80.34 |
6 |
- + + - + - - - |
50.87 |
1170 |
72.28 |
7 |
+ + - + - - - + |
59.18 |
1410 |
70.67 |
8 |
- - - - - - - - |
61.34 |
1130 |
81.23 |
9 |
+ - + + - + - - |
45.56 |
580 |
74.18 |
10 |
- + + + - + + - |
42.12 |
890 |
73.12 |
11 |
- - + + + - + + |
59.18 |
520 |
82.23 |
12 |
+ - + - - - + + |
29.35 |
470 |
71.73 |
Table 5 Outline and observed responses by Plackett–Burman Design
SOURCE |
DF |
SEQ SS |
ADJ SS |
ADJ MS |
F |
P |
Press Value |
|---|---|---|---|---|---|---|---|
Analysis of variance of product yield |
|||||||
Blocks |
1 |
130.42 |
9.992 |
9.992 |
0.23 |
0.0678 |
|
Main Effects |
8 |
507.77 |
507.771 |
63.771 |
1.48 |
0.0464 |
980.76 |
Residual Error |
2 |
85.55 |
85.552 |
42.776 |
|||
Total |
11 |
723.74 |
|||||
Analysis of variance of particle size |
|||||||
Blocks |
1 |
5.333 |
1.778 |
1.778 |
0.14 |
0.0742 |
|
Main Effects |
8 |
38.778 |
38.778 |
4.847 |
0.39 |
0.0862 |
760.65 |
Residual Error |
2 |
24.889 |
24.889 |
12.444 |
|||
Total |
11 |
69 |
|||||
Analysis of variance of %E.E |
|||||||
Blocks |
1 |
0.337 |
23.57 |
23.57 |
1.27 |
0.0377 |
|
Main Effects |
8 |
257.969 |
257.97 |
32.52 |
1.73 |
0.0417 |
879.348 |
Residual Error |
2 |
37.239 |
37.24 |
18.62 |
|||
Total |
11 |
295.545 |
|||||
Table 6 Summary of Analysis of variance for dependent variables
Influence of critical process parameters on quality of product
Percentage yield
Pareto chart: shown in Figure 1 influences that temperature and aspirator capacity has significant effect on %yield. Percentage yield was found to be in the range of 29-73%.
Contour plot: Two-dimensional contour plots presented in Figure 2 is useful to study the interaction effects of the factors on the responses. The relationship between the dependent and independent variables was elucidated by constructing response surface plots. ForY1 response i.e. % yield if, X2 from −1 to +1 level increased, % yield was increased and similar if X3 was decreased, % yield value was found to be increased.
(Product yield %) Y1=1.57X1+1.92X2+3.35X3-0.29X4+2.01X5-1.05X6-4.31X7-1.34X8Eq….4
Entrapment efficiency
Pareto chart: This shows the significant effect of inlet temperature during spray drying process over the entrapment efficiency. Figure 3 With increase in inlet temperature, entrapment efficiency was found to be increased. The temperature employed in the spray drying operation should be compatible with the material to be dried and the solvent used.
Contour plot: For the Y2 response percentage entrapment efficiency (%E.E), the interaction between factors X1 and X2 can be elucidated by using contour plot illustrated in Figure 4. When X1 from −1 to +1 level increased, % entrapment efficiency was increased and similarly if X2 was increased, entrapment efficiency value was found to be increased.
(% E. E)Y3=0.139X1+1.25X2-1.86X3+2.71X4-1.26X5-2.08X6+0.32X7-1.01X8 Eq…... 5
Particle size
Pareto chart: Figure 5 represented the effect of flow rate and inlet temperature significantly influences on the particle size of micelles product.
Contour plot: For the Y3response i.e. particle size shown in Figure 6 and as X1 from −1 to +1 level increased, particle size was found to be decreased and similarly when X2 was increased, particle size was found to be decreased.
(Particle size) Y2=12.50X1-0.66X2-0.66X3+0.33X4-0.6X5-1.16X6-0.83X7-0.16X8Eq……6
The coefficients of quadratic equations generated for individual responses by particle least square regression shown in tabular form Table 7. As feed flow rate decreases, the amount of solid in each droplet decreases at the nozzle. Therefore, when the solvent in the droplet evaporates, a smaller particle remains. Aspirator rate and inlet temperature are also significantly influences on particle size. The temperature employed in spray drying operation has to be compatible with the material to be dried and the solvent used. High pumping rates during the spray drying process results in large volumes of nebulizer solution to be dried.
Co-efficient |
% Yeild (Y1) |
Particle size (Y2) |
% E.E (Y3) |
A1X1 |
12.5 |
1.57 |
0.139 |
A2X2 |
-0.66 |
1.92 |
1.25 |
A3X3 |
-0.66 |
3.35 |
-1.86 |
A4X4 |
0.33 |
-0.29 |
2.71 |
A5X5 |
-0.6 |
2.01 |
-1.26 |
A6X6 |
-1.16 |
-1.05 |
-2.08 |
A7X7 |
-0.83 |
-4.31 |
0.32 |
A8X8 |
-0.16 |
-1.34 |
-1.01 |
Table 7 Coefficient of Quadratic equation for each independent variable
Optimization of parameters and validation of plackett-burman design
After generating the polynomial equations relating the dependent and independent variables, spray drying parameters were optimized for the responses. The optimum values for the variables were obtained by graphical and numerical analyses using the Minitab English 15 software which are based on criterion of desirability. Percentage error was measured so as to find out the optimized spray drying condition. As shown in, the observed value was found quite closer to the predicted value. Overlay plot shown in Figure 7 reveals the super imposable graph of individual contour plot of different responses with desired values, indicates that as aspirator capacity and temperature increases and as flow rate decreases particles having desired characteristic are formed.
For validation of results, the experimental values of the responses were compared with the anticipated values and the prediction error was found to vary between 2.3 and 6% shown in Table 8. The linear correlation plots drawn between the predicted and experimental values demonstrated high values of R2 (ranging between 0.9839 and 0.9957) indicating excellent goodness of fit (p<0.001). Thus the low magnitudes of error as well as the significant values of R2 in the present study prove high ability of Plackett-Burman Design.
Batch |
Independent Factor |
Opt value |
Dependent factor |
Predicted response |
Observed response |
% error |
PM |
Drug : Polymer Ratio |
2:01 |
% Product Yield |
69.8 |
69.75 |
0.071633 |
Ultra Sonication Time |
40 |
|||||
Ultrasonic Amplitude% |
80 |
|||||
Solvent (DCM: Ethanol) Ratio |
75:25:00 |
Particle Size |
130 |
132 |
1.28 |
|
Aspirator Capacity |
45 |
|||||
Feed Rate(ml/min) |
5 |
Table 8 Checkpoint batch, predicted and observed values of response variables and percentage predicted error
Table 8 Checkpoint batch predicted and observed values of response variables and percentage predicted error.
Characterization of spray dried polymeric particles
Particle size and zeta potential: Particle size was measured using Malvern zeta size and the average particle size (Figure 8) was found to be 95nm with polydispersity value 0.142 reveal narrow size distribution. Zeta potential was found to be -12mV which indicates electrostatic repulsion among particles and is enough for good stability.20
Transmission electron microscopy (TEM): The TEM micrographs shown in the Figure 9 where particles with uniform spherical shape and good with agreement with mean particle size measured by the Zetasizer.
Critical micelle concentration (CMC): CMC was determined at an absorbance of 519nm in aqueous solutions of the polymer derivatives against polymer concentration and observing the threshold above which particles form,21 presented in Figure 10, CMC was found to be 0.6mm.
Evaluation of spray dried polymeric nanoparticles
Drug release mechanism: Application of drug release data in model dependent and model independent approach. The drug release was found to be in the range of 74-98%. Also release kinetics shown in Table 9 describes the drug release mechanism from polymeric matrix. Korsmeyer-Peppas model describes the release mechanism of drug from matrix. Release profile of log fraction released versus log time was plotted and its slope value which is indicated by ‘n’ was calculated. It was found that n values were obtained in the range of 0.5-1 which indicates non-fickian diffusion. Maximum R2i.e; near to 1and minimum SSR value was found in case of Higuchi model which indicated that Higuchi was the best fitted model. Similarity factor and dissimilarity factor should be in the range of 50-100 and 1-15 respectively shown in Table 10. Similarity factor was found to be highest and dissimilarity factor was found to be lowest. Hence, batch 4 was found to be optimized according to drug release study.
Rifampicin |
Ethambutol |
Ofloxacin |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
n |
Intercept |
R² |
SSR |
n |
Intercept |
R² |
SSR |
N |
Intercept |
R² |
SSR |
||
batch 1 |
Zero order |
11.57 |
0.92 |
909.14 |
14.44 |
0.9 |
1014 |
9.82 |
0.95 |
319.8861 |
|||
First order |
0.097 |
0.84 |
12.58 |
1.037 |
0.55 |
1.7674 |
0.974 |
0.59 |
0.389351 |
||||
Higuchi |
0.9064 |
0.95 |
0.085 |
0.027 |
0.91 |
0.097 |
0.028 |
0.95 |
0.06153 |
||||
Kosmeyer Peppas |
0.56 |
0.572 |
0.76 |
0.43 |
0.56 |
0.54 |
0.77 |
0.3989 |
0.469 |
0.585 |
0.69 |
0.081641 |
|
batch 2 |
Zero order |
3.795 |
0.99 |
145.27 |
14.2 |
0.85 |
619.86 |
9.836 |
0.87 |
299.576 |
|||
First order |
0.862 |
0.7 |
1.99 |
1.02 |
0.48 |
1.615 |
0.942 |
0.5 |
0.31951 |
||||
Higuchi |
0.928 |
0.26 |
0.1483 |
0.068 |
0.96 |
0.059 |
0.052 |
0.89 |
0.09941 |
||||
Kosmeyer Pappas |
0.76 |
0.708 |
0.7 |
0.667 |
0.44 |
0.43 |
0.74 |
0.301 |
0.343 |
0.491 |
0.6 |
0.10233 |
|
batch 3 |
Zero order |
10.24 |
0.88 |
219.23 |
12.58 |
0.79 |
447.81 |
11.05 |
0.85 |
229.83 |
|||
First order |
0.925 |
0.49 |
2.5088 |
0.96 |
0.44 |
1.511 |
0.954 |
0.455 |
0.31848 |
||||
Higuchi |
0.086 |
0.96 |
0.0439 |
0.12 |
0.92 |
0.1117 |
0.123 |
0.95 |
0.05495 |
||||
Kosmeyer Pappas |
0.36 |
0.417 |
0.69 |
0.258 |
0.39 |
0.39 |
0.67 |
0.3248 |
0.3 |
0.375 |
0.69 |
0.06062 |
|
batch 4 |
Zero order |
23.34 |
0.77 |
4961.6 |
6.3 |
0.98 |
218.33 |
6.379 |
0.98 |
252.934 |
|||
First order |
1.121 |
0.52 |
2.4537 |
0.95 |
0.68 |
1.3226 |
0.951 |
0.67 |
0.44266 |
||||
Higuchi |
0.012 |
0.9 |
0.2059 |
0.1 |
0.93 |
0.877 |
0.106 |
0.93 |
0.09832 |
||||
Kosmeyer Peppas |
0.66 |
0.583 |
0.76 |
0.5809 |
0.59 |
0.71 |
0.71 |
35.206 |
0.5 |
0.712 |
0.71 |
0.1145 |
|
batch 5 |
Zero order |
10.29 |
0.95 |
635.64 |
11.51 |
0.89 |
1752 |
8.305 |
0.97 |
420.169 |
|||
First order |
0.955 |
0.61 |
1.637 |
0.92 |
0.61 |
1.7 |
0.939 |
0.64 |
0.53778 |
||||
Higuchi |
0.11 |
0.97 |
0.03 |
0.11 |
0.95 |
0.07 |
0.128 |
0.97 |
0.03083 |
||||
Kosmeyer Peppas |
0.3 |
0.83 |
0.18 |
1.67 |
0.83 |
0.62 |
0.77 |
0.1 |
0.7 |
0.61 |
0.77 |
0.05619 |
|
Table 9 Kinetics of drug release behaviour based on diffusion
f1 (Difference Factor) |
f2 (Similarity Factor) |
|||||
|---|---|---|---|---|---|---|
RIF |
EMB |
OFX |
RIF |
EMB |
OFX |
|
Batch 1 |
1.65 |
11.9 |
8.8 |
74.3 |
71.02 |
69.14 |
Batch 2 |
8.4 |
3.33 |
21.4 |
71.09 |
68.61 |
55.4 |
Batch3 |
24.17 |
14.9 |
21.7 |
54.39 |
59.02 |
55.35 |
Batch 4 |
0.74 |
2.85 |
4.9 |
92.7 |
79.15 |
79.8 |
Sol.eva |
43.9 |
56.03 |
37.7 |
45.22 |
37.65 |
46.7 |
Table 10 Similarity and difference factor of different batches
Aerodynamic behaviour study: The optimized formulation was subjected to in vitro lung deposition study using Anderson cascade impactor. Percentage emission was found to be 90%. After plotting graph as shown in Figure 11–13. Mass median aerodynamic Diameter and Geometric Standard Deviation was found to be 1.61μm and 2.32 respectively. Optimum range is defined as 0.5-5μm because particles <0.5μm are usually exhaled whereas particles >5.0μm are impacted in the oropharynx. Hence the powder is suitable for the delivery to the peripheral alveolar airway.
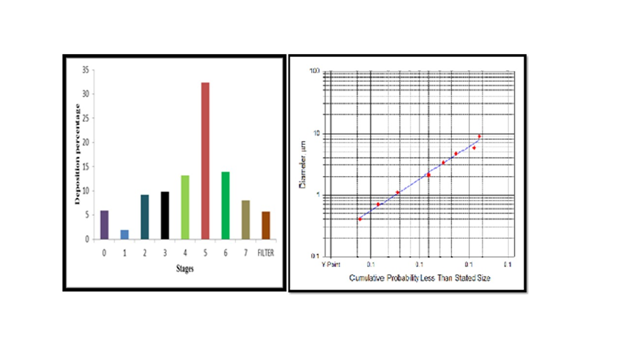
Figure 13 Bar graph representing a) amount of powder deposited at different stages b) Log probability graph.
Anti-microbial activity: Three-drug combinations tested may be useful against drug resistant isolates, although the combination including OFX showed better efficacy, being of potential use in drug-susceptible and INH-resistant isolates. The OFX, RIF and EMB combination showed significantly synergism (p 0.007) for drug-susceptible isolates shown in Table 11. Three-drug combinations tested may be useful against drug-resistant isolates, although the combination including OFX showed better efficacy, being of potential use in drug susceptible and INH-resistant isolates.22 Study of drug combinations with three drugs are probably a more realistic approach since most treatment regimens involve combinations of at least three anti-tuberculous drugs.
Isolates |
MIC in Alone (ug/ml) |
MIC in Combination (ug/ml) |
||||
|---|---|---|---|---|---|---|
RIF |
EMB |
OFX |
RIF |
EMB |
OFX |
|
1S |
0.05 |
2.5 |
0.05 |
0.06 |
0.31 |
0.03 |
2S |
0.05 |
2.5 |
0.05 |
0.06 |
0.31 |
0.03 |
3S |
0.05 |
2.5 |
0.012 |
0.06 |
0.31 |
0.03 |
4S |
0.05 |
2.5 |
0.025 |
0.06 |
0.31 |
0.03 |
5S |
0.05 |
2.5 |
0.025 |
0.06 |
0.31 |
0.03 |
6S |
0.05 |
2.5 |
0.025 |
0.06 |
0.31 |
0.03 |
7S |
0.05 |
2.5 |
0.025 |
0.06 |
0.31 |
0.03 |
8S |
0.05 |
2.5 |
0.05 |
0.06 |
0.31 |
0.03 |
9S |
0.05 |
2.5 |
0.05 |
0.06 |
0.31 |
0.03 |
10S |
0.05 |
2.5 |
0.05 |
0.06 |
0.31 |
0.03 |
H37Rv* |
0.05 |
2.5 |
0.025 |
0.06 |
0.31 |
0.03 |
Table 11 MICs of the OFX, EMB and RIF combination of the isolates studied
The cost of developing a new chemical entity is so much high that in the short-term the best option for novel anti-tubercular therapy is to re-formulate existing drugs to enhance their effectiveness and diminish their side effects can be overcome by developing an inhalable polymeric particle for anti-tubercular therapy. The method employed for the preparation of polymeric particles for inhalation was spray drying. Combinations of three drugs (Rifampicin, Ofloxacin and Ethambutol) and polymer (PLGA) were used. Optimized batch which can be effectively produced by spray drying technology. This engineered drug loaded polymeric particles could be used as an enhanced therapeutic alternative of the standard oral anti tubercular regimen, rescuing oral dosing, shortening drug regimen and limiting toxicity. Exploration polymeric particles as formulation address the challenging issues available formulation like good aerosol performance, capability of controlling and prolonging micelle uptake by alveolar cells of lung. Improvement in local bioavailability of drugs decreases resistance as site specific drug delivery. This will ultimately improve patient compliance and minimize the development of anti -tubercular resistance.
None.
The author declares no conflict of interest.

©2017 Patel. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.