Journal of
eISSN: 2376-0060


Research Article Volume 3 Issue 1
Pavlov First State Medical University of St Petersburg, Russia
Correspondence: Fedoseev GB, Pavlov First State Medical University of St Petersburg, Russia
Received: February 16, 2016 | Published: March 16, 2016
Citation: Fedoseev GB, Trofimov VI, Timchik VG, et al. Infectious and non-infectious sensitisation of patients with bronchial asthma and chronic obstructive pulmonary disease. J Lung Pulm Respir Res. 2016;3(1):31–39. DOI: 10.15406/jlprr.2016.03.00073
The study involved 169 people, of which 33 were practically healthy, 69 had bronchial asthma, 24 had bronchial asthma combined with chronic obstructive pulmonary disease, 35 had COPD and 8 had community-acquired pneumonia. We assessed the presence of IgE to tick allergens, dust allergens, and the combined allergens of grass, trees, weeds and flower pollen. We determined the presence of IgE and IgG to the allergens Strept. рneumon., Haemofil. influenzae, Neisseria рerflava and Staph. аureus. We assessed the presence, multiplicity, severity and combination of sensitisation to the presence of specialised IgE towards infectious and atopic allergens. All study groups displayed sensitisation, including heaslthy people and patients with COPD and community-acquired pneumonia, who did not show clinical allergy symptoms. A statistically significant direct correlation was established between IgG and IgE to Strept. Pneumoniae and Haemofilus influenza in healthy subjects and those with pulmonary conditions. The IgE or IgG reaction to Neisseria perflava and Staph. аureus did not show a significant correlation in either healthy or affected subjects.
Keywords: sensitization, allergy, mild bronchial asthma, immunoglobulin, allergen, antigen
Mod. BA, moderate bronchial asthma; Mod. BA+COPD, moderate bronchial asthma combined with chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; CAP, community-acquired pneumonia
Sensitisation means increased sensitivity, and presents as the main issue in our understanding of what is an allergy and allergic diseases, their aetiology, pathogenesis, diagnosis, clinical presentation, prevention and treatment. The study of sensitisation includes three main sub-groups: to what sensitisation occurs, the mechanisms creating sensitisation, and its consequences. Success in the study of this complex field of internal medicine can only be achieved via a systemic approach, where studies are carried out on the molecular, cellular, tissue, organ and whole body levels.1 The size of this journal article does not allow us to cover all aspects of this issue, so we will focus on only facts that are most closely to clinical medicine, and relate to the organ or systemic levels, especially because the end result of sensitisation is either good health or disease.
The susceptibility of patients with allergies to infection
Patients with bronchial asthma are more likely to develop diseases that are related to lower airway bacterial infections and viral respiratory infections.2 During the H1N1 influenza epidemic in 2009, patients with BA developed pneumonia twice as often as people without allergies, 50% and 27% respectively, and the pneumonia was significantly more severe, requiring ICU admission in 33% and 19% of patients, respectively.2 Infection with influenza in children with allergies during the 2009 epidemic was diagnosed much more frequently than in children without diagnosed allergies.3 The risk of developing severe bronchial asthma is particularly high when the child is exposed to airborne allergens and a viral infection.4
Patients with atopic BA are also highly susceptible to viral infections, especially by the rhinovirus.5 Patients with BA have an increased susceptibility to bacterial infections, particularly pneumococcal.6 The risk of invasive pneumococcal infection is higher in patients with severe BA, and reaches 4.2 episodes per 10,000 people per year, while it is 2.3 episodes in patients with moderate BA, and 1.2 episodes in patients with mild BA.7 BA is considered a risk factor for influenza and community-acquired pneumonia.8
Patients with BA are at an increased risk of getting mycoplasmal pneumonia9 and whooping cough.10 BA turned out to be the most common chronic condition among children affected by shingles.11 BA is one of the factors that promote the development of AIDS.12 One of the reasons that the presence of BA increases susceptibility to viral and bacterial infection is considered to be the innate or acquired interferon insufficiency,7,13 as well as immune incompetence of various nature and severity.14 Moreover, the increased susceptibility of BA patients to infections is related to defects in their epithelial barrier function. The susceptibility of patients with allergic conditions to infections, which is related to the incompetence of their immunological defence, justifies the need for considering the appearance of systemic disorders in this group of patients.15 One of the treatment methods to be discussed is the use of vaccines in this group of patients.
Some points relating to atopic (non-infectious) allergies
There is published data available on dozens of different airborne, food, industry, drug-related and other allergens, and every year new names are added to this list. The 15 most clinically common allergens are considered to be the main ones with diagnostic complexes being created for sensitisation diagnosis, which use specialised IgE. Using skin samples with airborne allergens, the rates of sensitisation in patients with BA varies between 51% and 81.9%.16 The main fundamental mechanism of sensitisation is the formation of specialised IgE.17 There is a correlation between levels of specialised IgE, total IgE, the results of skin allergy testing, and BA severity.18 Patients with mild and moderate BA have a significant correlation between levels of specialised IgE to non-infectious allergens and the results of skin tests, the acetylcholine inhalation test, and the exhaled nitric oxide content.19
It is important to note that sensitisation and the related appearance of antigen-specific IgE antibodies is not necessarily accompanied by clinical allergy symptoms.20 Therefore, sensitisation that manifests as an increased content of specialised class IgE antibodies can occur in people without allergy symptoms, including practically healthy people, as well as in people with allergies. An allergy is the combination of sensitisation (increased number of antigen-specific IgE antibodies) and clinical signs of an allergic condition.21
The sensitivity to airborne allergens does not decrease with time.19 The biochemical properties of allergens and the sensitivity of respiratory epithelium to their active components can increase the permeability of the respiratory mucous membrane to respiratory allergens. Many allergens contain serine, cysteine and other proteases, which disrupt the barrier function of the respiratory epithelium and increase the allergenic effect of respiratory allergens.22
Th1, Th2 and allergic diseases
The most widely accepted current hypothesis of the pathogenesis of BA and other allergic diseases is the idea that they are related to the predominance of the Th2 cytokine system. Newborns had a predominance of Th2 activity of the immune system,23 then the influence of the gut micro biota stimulates the development of Th1 cells, which forms immune tolerance and protects from the possible development of atopic diseases and BA.24 The effect of external factors, such as dust, dust mites, pollen, and microbial endotoxin, leads to the differentiation of pro-inflammatory Th2 cells. This is accompanied by the expression of pro-inflammatory cytokines IL-4, IL-5, IL-9 and IL-1325 and IgE synthesis, an increase in the activity of eosinophils and mast cells, with sensitisation and the appearance of clinical signs of allergy.26 Th1 cells participate in the formation of delayed hypersensitivity reactions and inhibit Th2-controlled processes.26 Respiratory viral infections stimulate Th1 activity and increase interferon activity.27 Nasal cavity colonisation with Staphylococcus aureus is accompanied by local Th2 inflammation. With a predominance of Treg cells activity, Th2 cell proliferation ceases, there is formation of airborne allergen tolerance and the synthesis of specialised IgE is slowed down.28
Th2 system activity leads to fibro proliferative changes in the lungs. Granulomatous inflammation is characteristic of Th1.29
Th2 system activity cannot fully explain the pathogenesis of atopy and BA.30 Bronchial hyperactivity and increased mucus secretion can occur without IgE and eosinophilia in the bronchial contents. Treatment directed at selectively blocking Th2 activity has been ineffective.31 There is data that points towards the role of allergen-specific IgG in Th2-mediated allergic diseases, such as BA, bronchial aspergillosis and allergic alveolitis.32
Apart from the Th1 and Th2 cellular immune balance, there is a Th17 cell system, which is mostly present in the intestinal mucosal lining.24 This cell system is involved in the body’s defence against microbes and affects the appearance and course of allergies and BA.33 One of the reasons for neutrophilic inflammation is thought to be endotoxin-containing dust particles, whose inhalation is accompanied by increased activity of IL-1 and TNFα, and neutrophilic inflammation.34 Prevention and treatment of allergic diseases and BA depends on the effect on the immunological regulation and the function of the immune system.35 Clinical interpretation of IgE levels in blood serum.
Increased levels of specialised IgE in patients with atopic diseases, such as atopic BA, atopic dermatitis and allergic rhinitis, are a symptom of sensitization.36 It is important to note the difference between sensitisation and allergy. Sensitisation is an increase in the levels of specialised class E antibodies, but at the same time, contact with a particular allergen does not trigger symptoms of allergic disease. In the presence of an allergy, contact with a sensitising allergen is accompanied by the appearance of corresponding symptoms.21 The cellular and sub cellular processes of allergic reaction have been studied in detail, and the order of events following the binding of an allergen to two neighbouring specialised IgE’s on the surface of mast cells and basophils is well known, involving the release of pro-inflammatory mediators, such as histamine, tryptase, leukotriene and prostaglandin, and the ensuing development of clinical symptoms.26
Bacterial IgE titres reflect the presence and degree of impact of the bacterial antigen.37 The level of total blood serum IgE is related to the features of BA progression, but is not related to specific allergic sensitisation. A reduction in total blood plasma IgE levels correlates with an improvement in the clinical progression of BA.38 An increased level of total blood plasma IgE corresponds to BA severity is linked to eosinophilia and a higher content of eosinophilic cationic protein in the blood. IgE content on the surface of respiratory organ mucous membranes is not related to the systemic levels of IgE, but is regulated by the local reactivity of the mucosa, which depends on the effect of antigens. The local function of IgE is a homeostatic protective mechanism of the respiratory mucous membrane, in both healthy subjects and those with BA.39
Increased levels of total IgE have been noted in cases of parasitic disease and Bronchopulmonary aspergillosis.40
BA and other allergic diseases can be divided into two types: the external, allergic version related to the sensitisation to allergens in the external environment and accompanied by increased blood plasma IgE levels, and the internal, non-allergic version, without sensitisation and a low level of blood plasma IgE.41 70%-90% of BA cases are related to IgE-dependent mechanisms.42 An allergen-specific IgE against microbial components has been found in 50% of patients with BA. The obtained data allows us to hypothesise that in these patients, microbial sensitisation is a trigger for BA of this phenotype.41 IgE antibodies to Pneumococcus43 and to Staphylococcus aureus endotoxin44 have been found in the blood serum of patients with BA.
In recent years, there has been an increased interest in opportunistic microorganisms. Allergies to Neisseria and Staphylococcus aureus were confirmed in patients with BA in our study: IgE isotype antibodies to Staphylococcus aureus were found in 69.2% of patients with BA, and to Neisseria in 85.7% of patients.45 IgE to the respiratory syncytial virus were found in the blood plasma of children with bronchiolitis, with increased levels being accompanied by the appearance of wheezing.43
In mouse experiments, specific antiviral E antibodies were obtained.46 However, we must emphasise that there is no basis for considering antiviral IgE as being significant during the development of allergic disease that is virus-related.47
IgE auto-antibodies against human tissue have been found in the blood serum of patients with allergic BA. It is hypothesised that IgE auto-antibodies against human tissue create more severe BA.41
Clinical interpretation of IgG levels in blood plasma
IgG molecules have a unique ability to create both inflammatory and anti-inflammatory reactions.48 IgG is involved in the neutralisation of toxins and viruses, and protects the body from various microorganisms.48
Concentrations of IgG1, IgG2, IgG3 and total IgG are reduced in young children with BA, which is related to a delay in the maturation of the immune system and plays a role in the pathogenesis of BA. Children of any age with BA have a lower level of IgA and IgG, with the IgG3 subclass having the most deficits.49 Adults with BA have been found to have specific IgG to the Aspergillus fumigatus antigen, which is seen as a manifestation of the aetiological significance of this microorganism.50
Due to the high level of IgG antibodies to Chlamydia pneumoniae in patients with non-atopic BA, a hypothesis was made about the causal significance of this microorganism and BA. However, a comparison of the specific IgE content and the release of histamine from basophils in these patients allowed researchers to discard this theory.50
The anti-inflammatory effect of IgG has been shown both experimentally and clinically. It has been established that specific IgG can block the binding of specialised IgE with antigens and thus reduce the hypersensitivity and inflammation in patients with BA.32
The aim of this study was to investigate the non-infectious (atopic) and infectious (bacterial) sensitisation in people with clinical symptoms of allergy, and people without symptoms, using specific IgE for atopic and specialised IgE and IgG for infectious allergens.
The aims of this study
The study included 169 people, of whom 33 were practically healthy (control group), 29 patients had mild bronchial asthma (1st group), 40 patients had moderate bronchial asthma (2nd group), 24 patients had moderate bronchial asthma as well as chronic obstructive pulmonary disease (3rd group), 35 patients had chronic obstructive pulmonary disease (4th group), and 8 patients had community-acquired pneumonia (5th group). The patient age and gender is presented in Table 1.
Study groups |
n |
Mean age М±s |
Men %% |
Women %% |
Healthy subjects |
33 |
49.39±21.02 |
33.3 |
66.7 |
Mild BA |
29 |
31.76±10.46 |
24.1 |
75.9 |
Moderate BA |
40 |
44.35±16.92 |
30 |
70 |
Mod. BA+COPD |
24 |
60.13±10.59 |
66.7 |
33.3 |
COPD |
35 |
64.77±8.37 |
20 |
80 |
CAP |
8 |
46.13±12.93 |
50 |
50.5 |
Table 1 Patient characteristics according to age and gender
The subjects underwent general clinical, laboratory and instrumental tests. Allergen-specific IgE and IgG antibodies in blood serum were determined using the immunoenzyme technique. A set of reagents was used for immunoenzymatic determination of allergen-specific IgE and IgG antibodies in blood plasma, using the solid-phase non-competitive indirect enzyme immunoassay. We assessed the presence of IgE to tick allergens, dust allergens, and the combined allergens of grass, trees, weeds and flower pollen. We determined the presence of IgE and IgG to the allergens Strept. рneumon., Haemofil. influenzae, Neisseria рerflava and Staph. аureus.
The program STATISTICA 6.1 (Stat Soft, Inc.) was used for statistical data analysis. The critical level of significance was considered to be 0.05 when interpreting the results. Descriptive statistics for quantitative signs used the mean and root-mean-square deviation (М±s). The Student t-test and Pearson’s correlation coefficient was also used.
It is worth noting the high frequency of sensitisation to the Staphylococcus aureus allergen in healthy and affected subjects.
Non-Infectious Allergens |
||||||
Allergens |
Healthy Subjects |
M Mild BA |
Moderate BA |
Mod. BA+COPD |
COPD |
CAP |
n=32 |
n=28 |
n=39 |
n=23 |
n=35 |
n=7 |
|
Ticks |
34.3 |
32.1 |
20.5 |
39.1 |
33 |
14.2 |
Dust |
34.3 |
42.2 |
33.3 |
26.1 |
38.8 |
42.2 |
Grass |
21.8 |
7.8 |
15.4 |
30.4 |
27.7 |
14.2 |
Trees |
40.6 |
32.1 |
20.5 |
39.1 |
38.8 |
14.2 |
Weeds |
25.1 |
21.4 |
17.9 |
23.1 |
38.6 |
28.6 |
Flowers |
40.6 |
42.8 |
23.1 |
30.4 |
50 |
14.2 |
Infectious Allergens |
||||||
Allergens |
n=29 |
n=28 |
n=31 |
n=9 |
n=35 |
n=8 |
S. Pneumonia |
51.7 |
28.6 |
54.8 |
33.3 |
58.3 |
61.5 |
Haemofilus Infl. |
58.6 |
53.6 |
67.8 |
66.6 |
55.5 |
61.6 |
Neisseria Perfl. |
55.2 |
46.4 |
67.7 |
22.2 |
61.1 |
30.8 |
Staph. Аureus |
93.1 |
92.8 |
87.1 |
77.8 |
83.3 |
100 |
Table 2 The percentage of study subjects with increased levels (class 2 or more) of IgE to non-infectious (atopic) and infectious allergens in blood serum, in healthy and affected people

Figure 1 The multiplicity of infectious and non-infectious sensitisation according to specific IgE levels (+1 or more) in %% as compared to the number of study subjects.
The data obtained from healthy subjects does not greatly vary from the results of the study patients. All the study subjects (apart from one patient with mild BA) had sensitisation to non-infectious allergens. All the study subjects predominantly had sensitisation to 4 or more non-infectious allergens. Sensitisation to 4 or more non-infectious allergens was noted to be 3 times more common than sensitisation to infectious allergens. With any number of significant allergens in healthy and affected subjects, there was a predominance of sensitisation to non-infectious allergens.
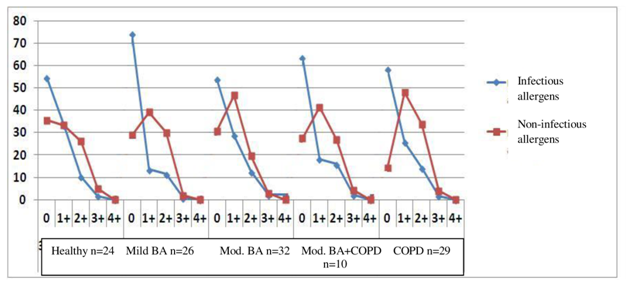
Figure 2 The degree of infectious and non-infectious sensitisation according to specific IgE levels in %% as compared to the number of study subjects.
When there was a lack of sensitisation (“0”), studies with infectious allergens predominated by two or more times, and the lack of infectious sensitisation was noted much more frequently that non-infectious sensitisation in healthy and affected subjects in all groups. Significant sensitisation to 4+ infectious and non-infectious allergens was almost wholly lacking with any degree of sensitisation in healthy and affected subjects in all study groups, the sensitisation to non-infectious allergens predominated.

Figure 3 The combination of infectious and non-infectious sensitisation according to the number of study subjects with varying combinations of specific IgE numbers (+1 or more) in %% as compared to the study subjects in the particular group.
Among the study subjects, the most common were patients from Group 2 (many non-infectious and few infectious IgE), rare were patients from Group 4 (many non-infectious and infectious IgE), and even rarer were patients from Group 1 (few non-infectious and infectious IgE), while there were almost no patients in Group 3 (few non-infectious and many infectious IgE). All groups that were characterised by a combination of sensitisation had both healthy subjects and patients with different disease entities.

Figure 4 The matrix for combination analysis of different levels of IgG and IgE to bacterial antigens in healthy and affected subjects
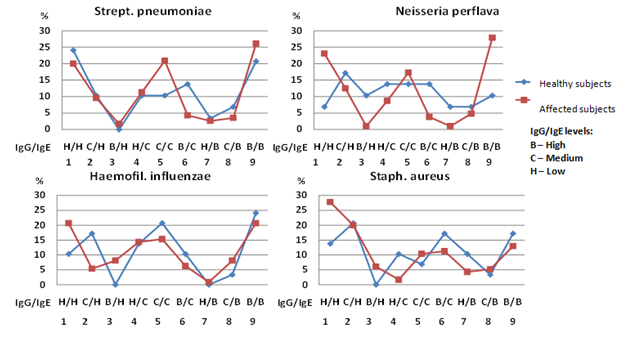
Figure 5 Combination versions of IgG and IgE to bacterial antigens in healthy subjects (n=29) and patients with BA and COPD (n=115) as a percentage of the total number of subjects in that group.
There is almost complete matching of data from healthy and affected subjects, obtained when determining IgG and IgE to Strept. рneumoniae. The most common combination of studied immunoglobulins is when they are numerous (version 1) or few (version 9). The rarest obtained combinations are ones where there is a lot of one immunoglobulin but not much of the other (version 3 and 7). Similar results were obtained when studying IgG and IgE to Haemofilus influenzaе. There was an absence of interaction when analysing IgG and IgE results to Neisseria perflava and Staph. аureus in healthy and affected subjects. The correlation between immunoglobulins to Neisseria perflava in healthy subjects was noted to be very consistent, with a lack of situations described above, where both immunoglobulins display increased or decreased levels.
The statistical significance of the correlation between IgG and IgE to bacteria in healthy and affected subjects is presented in Table 3.
IgG |
|||||
Healthy Subjects |
|||||
IgE |
Strept. Pneumon |
Haemofil Influenzae |
Neisseria Perflava |
Staph. Aureus |
|
Strept. Pneumon |
0.6744 |
0.489 |
0.3405 |
0.2321 |
|
N=29 |
N=29 |
N=27 |
N=29 |
||
р=.000 |
р=.007 |
р=.082 |
р=.226 |
||
Haemofil Influenzae |
0.5178 |
0.686 |
0.4012 |
0.3486 |
|
N=29 |
N=29 |
N=27 |
N=29 |
||
р=.004 |
р=.000 |
р=.038 |
р=.064 |
||
Neisseria Perflava |
0.2711 |
0.3604 |
0.1475 |
0.2641 |
|
N=29 |
N=29 |
N=27 |
N=29 |
||
р=.155 |
р=.055 |
р=.463 |
р=.166 |
||
Staph. Aureus |
-0.2988 |
-0.2708 |
-0.5332 |
0.4398 |
|
N=29 |
N=29 |
N=27 |
N=29 |
||
р=.115 |
р=.155 |
р=.004 |
р=.017 |
||
Affected Subjects |
|||||
IgE |
Strept. Pneumon |
0.7506 |
0.7506 |
0.6417 |
-0.0429 |
N=115 |
N=104 |
N=104 |
N=115 |
||
P=.000 |
P=.000 |
P=.000 |
P=.649 |
||
Haemofil Influenzae |
0.2285 |
0.4751 |
0.4098 |
-0.011 |
|
N=115 |
N=104 |
N=104 |
N=115 |
||
P=.000 |
P=.000 |
P=.000 |
P=.908 |
||
Neisseria Perflava |
0.7399 |
0.62 |
0.8123 |
-0.011 |
|
N=115 |
N=111 |
N=104 |
N=115 |
||
P=.000 |
P=.000 |
р=.000 |
P=.908 |
||
Staph. Aureus |
0.065 |
0.0423 |
0.1078 |
0.2621 |
|
N=115 |
N=111 |
N=104 |
N=115 |
||
р=.490 |
р=.659 |
р=.276 |
P=.005 |
||
Table 3 The correlation between IgG and IgE content to bacteria in healthy subjects and patients with BA and COPD
It is worth noting the statistically significant direct correlation between IgG and IgE to Strept. pneumoniae and Haemofilus influenzae. There is a correlation between these immunoglobulins not only for specific bacteria, but even for different bacteria. IgG to Haemofilus influenzae not only correlates with IgE to Haemofilus influenzae, but also with IgE to Strept. рneumoniae, while IgG to Strept. рneumoniae correlates not only with IgE to Strept. рneumoniae, but also with IgE to Haemofilus influenza. Patients with various diseases had a stronger statistically significant correlation between IgG and IgE to Strept. рneumoniae, Haemofilus influenza and Neisseria perflava, than healthy subjects. Other data was obtained when studying immunoglobulins to Staph. aureus: there was only a statistically significant correlation between IgG and IgE to Staph. aureus, with no significant link between IgG to Staph. aureus and IgE to other bacteria. We did not find a similar link between IgG and IgE to bacteria, in the available literature.
The obtained data allows us to hypothesise about the presence of coordinated actions by the macroorganism to protect itself against pathogenic respiratory bacterial micro flora (Strept. рneumoniae and Haemofilus influenza IgE and IgG). The response of the macro organism to the activity of non-pathogenic and opportunistic micro flora is not accompanied by complete coordination (Neisseria perflata and Staph. Aureus IgE and IgG). Further studies are required for the pathogenetic and clinical interpretation of the ascertained fact of coordination between IgG and IgE to bacterial antigens.
Questions that arise as a result of studying atopic and infectious sensitisation
None.
The author declares no conflict of interest.

©2016 Fedoseev, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.
World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.