Journal of
eISSN: 2373-4345


Research Article Volume 3 Issue 1
1Private practice, Brazil
2Department of Prosthodontics, University of Tennessee Health Sciences College of Dentistry, USA
3Department of Periodontology, Federal University of Santa Catarina, Brazil
Correspondence: Cimara Fortes Ferreira, Department of Periodontology, University of Tennessee Health Sciences College of Dentistry, USA, Tel (901) 448-6751
Received: October 06, 2015 | Published: October 27, 2015
Citation: Buttendorf AR, Ferreira CF, Bianchini MA, et al. Minimal time for gingival retraction cords to achieve accurate impression: an in vivo study. J Dent Health Oral Disord Ther. 2015;3(1):244-248. DOI: 10.15406/jdhodt.2015.03.00078
Aim: Analyze minimal time interval necessary for retraction cords (RC) embedded in 4% L-epinephrine (LE) or 25% aluminum chloride (AC) to expose cervical finish line of tooth preparation for satisfactory impression without causing gingival recession (GR).
Method: Cords were placed in 80 teeth of 5 mongrel dogs divided into 3 groups:
30 teeth received LE cords.
30 teeth received SC cords.
20 controls did not receive RC.
Cords stayed in sulcus for 3, 7, and 15 minutes and potential GR analyzed at 7 and 14 days.
Results: Groups 1 & 2 showed 100% and the group 3, 35% of satisfactory gingival displacement (GD) (P=0.05). Groups 1 & 2 showed similar GD in all time intervals. When the RC was used over 3 minutes, in groups 1 & 2, GR occurred.
Conclusion: Placement of cords soaked in 4% LE or in 25% AC, for up to 3 minutes in the gingival sulcus, does not cause GR.
Keywords: retraction cords, gingival recession, retraction agents, L-epinephrine, aluminum chloride
The introduction of elastomeric impression materials in dentistry resulted in the development of different methods of tissue retraction in order to achieve accurate impression of the finish line of the tooth preparation, which is necessary to fabricate a prosthetic crown. This area needs to be clean, dry and visible. The gingival retraction method1 is conducted using a retraction cord soaked in epinephrine or in an astringent agent. In addition to retracting the tissues laterally to permit impression taking of the tooth preparation finish line, the use of a retractor cord promotes a dry, clean gingival crevice, eliminating blood and/or gingival crevicular fluid.
The use of cords soaked in chemical substances has been indicated to promote local irreversible alteration in soft tissues,2-5 and also cause lysis of fibroblasts.6,7
There is a direct relationship between the time that the gingival retractor cord remains inside the sulcus and possible gingival recession, due to the fact that the junctional epithelium is affected the most vulnerable of the structures that protect the periodontium.8
The material used for gingival retraction must fulfill the following guidelines: be effective in lateral and vertical retraction of the gingival crevice in order to permit an adequate volume of impression material; not damage the tissues irreversibly (it should be understood that even the most meticulous method results in some degree of tissue damage); result in a reversible damage and complete clinical and histological healing within two weeks; not have harmful systemic effects; have enough time to retract the sulcus in order to permit an accurate impression.9 In addition, the gingival retraction material should be efficient in a short period of time.10 Retraction materials should control the crevicular fluid and gingival bleeding especially when elastomeric materials are used for the impression.4
The objective of this study was to conduct an in vivo clinical comparative analysis of the optimal period of time for gingival retraction cords, soaked in 4% L-epinephrine or alternatively 25% aluminum chloride, to stay in the gingival crevice in order for accurate impression of the cervical finish line of the tooth preparation without causing gingival recession.
Experimental pattern
The patterns of the study were: 5 male mongrel dogs, from 2 to 4 years of age, with a weight ranging from 12 to 18 kg, presenting all permanent dentition and free from cavities. This study was approved by the Ethics in Animals Research Committee.
The inclusion criteria for the study were the following: dogs presenting the third incisors, second premolars, third premolars, and fourth premolars in the upper arch; dogs presenting the second premolars, third premolars, fourth premolars, and first molars in the lower arch. A total of 80 teeth were divided into 3 groups: Group 1 comprised of 30 teeth which received cords soaked in epinephrine; Group 2 comprised of 30 teeth which received cords soaked in aluminum chloride; and, Group 3 comprised of 20 teeth which were the control group, which did not receive a retraction cord. It was imperative that the teeth selected for this study had a buccal keratinized gingival margin of at least 5 mm in width and thick gingival margin (the metal of the periodontal probe should not be seen through the gingival during probing). Thin gingival margin tissues, even when keratinized, show dubious capacity to resist intrasulcular procedures.4
Dental prophylaxis
Thirty days prior to the experiment, daily dental prophylaxis was conducted using the modified Stillman brushing technique by adapting to the anatomical tooth features of the animal with a soft toothbrush (Oral B – P35, São Paulo, SP, Brazil). As an adjunct procedure, topic 0.12% chlohexidine gluconate (PerioGard; Colgate-Palmolive, São Paulo, SP, Brazil) was applied. In order to eliminate and prevent periodontal diseases, manual scaling and tooth polishing were performed every fifteen days. This pre-treatment phase was enables by anesthetizing the animals with 2% chloridrate xilazin (Rompun-Bayer, Porto Alegre, RS, Brazil).
Absorbance of the retraction cord
After the 30 day control period, standard Ultrapak 00 non-soaked (Ultradent; South Jordan, UT, USA) retraction cords were placed in the sulcus of the selected teeth. The cords were soaked in 4% L-epinephrine or 25% aluminum chloride3 using the soaking time intervals described by Csempesz.11
Laboratory examinations were performed to confirm soaking of the cords. The aluminum chloride retraction cord absorbance was determined by the atomic absorption spectrophotometer model 3110 attached to 3 graphite furnace model HEA-600 (PerkinElmer; Boston, MA, USA). In order to compare the absorbance capacity of each medicament solutions, 3 pieces of 2cm length cords were previously soaked in 4% L-epinephrine or 25% aluminum chloride and further analyzed (Septodont; Saint-Maur des Fosses, France). Six pieces of cords were placed in different recipients containing 10mL of concentrated nitrous oxide for aluminum extraction solutions. The solution temperature was raised to 120°C for 60 minutes, evaporated and the recipient was washed with 10mL of nitrous oxide 1% (v/v). Such solutions were then analyzed in the atomic absorption spectrophotometer.
The quantity of L-epinephrine absorbed by the cord was determined by dosing ultraviolet spectrophotometric (2796nm) in the spectrophotometer UV/Vis model UV – 1601 (Shimadzu, Tokio, Japan) and by using 1 cm optical way quartz cells. The standard deviation was built using L-epinephrine bitartrate (Ariston, São Paulo, São Paulo, Brasil) in distilled water as a reference substance, showing a correlation coefficient of 1. The fragments of the cord soaked in 4% L-epinephrine were submitted to extraction with distilled water in a 10 mL volumetric balloon under flutter in standard temperature for 2 hours. The fragments were taken out from the balloon and submitted to new cycles of water extraction, the solvent was replaced 3 times and the time interval was every 30 minutes. The extracted liquids were submitted to spectrophotometric analysis (279,6nm) to determine the quantity of extracted L-epinephrine by inserting the absorbing values of the standard deviation.
Measurement
A compass (Trident, 9020, São Paulo, São Paulo, Brazil) with two spikes in the same shank was used to measure the possible gingival recession caused by the retraction cord. Such spikes in the same shank was fixed in a cavity made at the buccal surface of the teeth crowns, with a laminate drill diameter ¼, (KG Sorensen; São Paulo, São Paulo, Brazil) which was the first measurement (Figure 1). The measurements were taken from the compass which had a digital caliper (model 797B Starret; Itu, São Paulo, Brazil) adapted to.
After taking the first measurements, the cervical cavities of the 4 teeth were prepared. The purpose of the cervical preparation was to characterize a reference point for the impression taking procedure. Therefore, a 0.8mm bur diamond round (KG Sorensen; São Paulo, São Paulo, Brazil) was used at the gingival level on the buccal aspect in order to avoid damage to the epithelium of the gingival crevice. After preparation and measurement procedures were conducted, the 3 teeth of the same semi-arch received a retraction cord that was placed with caution to avoid gingival tissue damage (Figure 2).
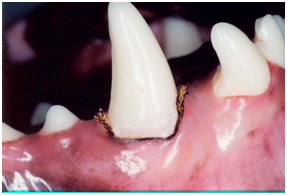
Figure 2 After preparation and measurement procedures were conducted, the 3 teeth of the same semi-arch received a retraction cord that was placed with caution to avoid gingival tissue damage.
Cord placement
Each dog was divided into 4 quadrants. Each quadrant received a different type of cord (Group 1 or Group 2) for the time intervals of 3, 7, and 15 minutes in the gingival crevice of each tooth. A fourth tooth was selected as control.
Turns were always taken among the teeth in order for all the teeth to receive the different types of cord uniformly. The placement technique and the position of the cord inside the gingival crevice were as described by Fischer.12 In each quadrant three cords were applied. The first cord was applied for 15 minutes. After 7 minutes, a second cord was introduced in the gingival crevice of the second tooth. The third cord was applied 11 minutes after the first one had been inserted.
Gingival Retraction Analysis
After removing the pre-soaked cord, a simultaneous impression technique was performed with a custom-made tray and a condensation cured silicone (Silon2 APS, Dentsply; Petrópolis, Rio de Janeiro, Brazil). After impression taking, all the teeth were restored with composite resin (Z100, 3M ESPE; Ribeirão Preto, São Paulo, Brazil). The impression was made and the special Type IV plaster was poured (Durone, Dentsply; Petrópolis, Rio de Janeiro, Brazil). The quality of gingival retraction was evaluated with the study models. The gingival retraction was considered sufficient when there was 0.3 mm or more of tooth structure under the finishing line of the tooth preparation. When the measurement was less than 0.3 mm, the retraction was considered insufficient (Figure 3). After impression taking, all the 4 teeth were restored with composite resin (Z100, 3M ESPE; Ribeirão Preto, São Paulo, Brazil). Care was taken during restorative and polishing procedures in order to avoid trauma to the adjacent gingival tissues.
The distances from the marginal gingiva and the reference marks were taken 7 and 14 days later, by means of the compass adapted to the digital caliper as described preciously.
The aim of the control group was to verify the possibility of gingival displacement without a retraction cord, namely; to have a group to compare the effectiveness of the gingival displacement achieved by both experimental groups. In addition, the control group enabled to verify potential gingival recession caused by other procedures that were inherent to the procedures necessary for a prosthetic fabrication. These procedures are: tooth finish line preparation; restorative, polishing, and finishing procedures; and, impression taking. This would enable the comparisons not to be influenced by gingival recessions caused by these procedures, and thus, eliminate the gingival recession factor caused exclusively by the use of the retraction cords in both experimental groups.
Statistical analysis
Duncan’s statistical test was applied to the results in the statistical significance interval of P=0.05.
All the groups, in both experimental groups, in all periods of analysis, showed satisfactory gingival displacement. The control group showed 7 working casts (35%) with satisfactory gingival displacement and 13 working casts (65%) with unsatisfactory gingival displacement.
The values for the gingival recession are statistically similar amongst the two experimental groups in all the time intervals. Both experimental groups at the time intervals of 3 or 7 minutes showed an average gingival recession of 0.3 mm, and at the 15-minute interval, showed an average gingival recession of 0.5 mm. For the experimental group receiving the retraction cord soaked in epinephrine there was a statistically significant increased in the gingival recession for the 15-minute time interval, when compared to the 3 and 7-minute time intervals. For the experimental group receiving the retraction cord soaked in aluminum chloride, there was also a statistically significant increased in the gingival recession for the 15-minute time interval, when compared to the 3 and 7-minute time intervals. In addition, the experimental groups showed statistical difference when compared to the control group (Table 1 & Graph 1). The results showed that the 7-day analyses were statistically similar to the ones showed in the 14-day analyses.
|
3 MINUTES |
|
7 MINUTES |
|
15 MINUTES |
|
|||
|
Mean Value |
Standard Deviation |
Duncan's Statistical Test |
Mean Value |
Standard Deviation |
Duncan's Statistical Test |
Mean Value |
Standard Deviation |
Duncan's Statistical Test |
Epinephrine |
0.339 |
0.17 |
a B |
0.39 |
0.2 |
a B |
0.552 |
0.29 |
a A |
Aluminum Chloride |
0.37 |
0.11 |
a B |
0.39 |
0.21 |
a B |
0.543 |
0.24 |
a B |
Control |
0.143 |
0.035 |
b A |
0.143 |
0.035 |
b A |
0.143 |
0.035 |
b A |
Table 1 Average Gingival recession difference in groups
There were no studies found in the literature that defined the exact time necessary for satisfactory gingival displacement without causing irreversible gingival recession. The present study concurs with the results shown in the literature2,13-15 that did not show gingival recession 14 days after retraction cord placement, when the cord remained in the crevicular sulcus for 10 minutes. A study showed that when the cord remained in the crevicular sulcus for 5 minutes in the 14-day analysis there was absence of gingival recession.5
The authors suggest that the results of this study differ from the reviewed literature due to the lack of meticulously detailed techniques employed for gingival retraction in other studies. This information could result in important criteria and alter the results of the research. These criteria could be as follows:
Pre experimental dental prophylaxis
Some studies2,5,13-15 did not conduct dental prophylaxis in the dogs in order to observe the total absence of gingival marginal inflammation. Therefore, the gingival retraction cord could have caused fewer traumas in the inflamed tissue due to a wider space for the placement of the cord. A healthy tissue presents a shallow gingival crevice. Inflamed tissues are not sufficiently resistant to the retraction cord and consequently the cord will cause less damage to the soft tissues. In this case there will be fewer traumas due to the unnecessity of disturbing the union enamel/crevicular epithelium. However, it is not advantageous to have inflamed tissue in order to have fewer traumas. Tissue inflammation signs are characteristics of injury and can result in tissue recession. Gingival recession can be unexpected when the gingival complex is affected by periodontal disease. Healthy gingival tissues are stable and less vulnerable to retraction.16
Retraction Cord Selection
Some studies2,5,13-15 did not standardized the thickness of the gingival retraction cords which disables comparisons amongst studies. It is important to take into account that the thicker the cord the higher the possibility of damage to the gingival tissues. Another important variable that was not analyzed in the studies mentioned above was the absence of the impression procedure and creation of a study model in order to verify if the retraction achieved by the cord was enough for obtaining a satisfactory study model. In addition, the studies mentioned above did not attempt to verify the absorbance capacity of the retraction cords. The presence of chemical substances in the cords is a fact that can increase local inflammatory response6 as a consequence to marginal tissue recession. The present study used the same concentration of aluminum chloride and L-epinephrine used in the cords of Donovan et al.9
Cord Placement Depth
The present study concurs with the literature when it shows that that gingival recession was higher due to the deeper insertion of the retraction cord.8,17 The retraction cord was not excessively tight to the point of covering the gingiva. Consequently, the cord remained positioned under the cervical finish line of the tooth preparation,12 remaining visible.1
Clinical Evaluation Comparison of the Gingival Tissue
The clinical evaluation of this study can be questioned by many other authors5,14,15 evaluated gingival retraction using a fixed reference point or the margin of the tooth at the free gingival crest. Such analysis can show inaccuracies due to lack of standardized technique when repeated measures are taken. In order to standardize the technique, in the present study, a three spikes compass was used to conduct standard measurements.
Result Analysis
Gingival recession values of 0.143mm presented in the control group are significant and can impair gingival esthetics and function.9 Such gingival recession can be caused by brushing trauma and scaling during the experimental period and/or trauma during impression taking. However, the brushing pressure was sufficient for food debris removal and the crown scaling in conjunction with tooth polishing did not reach gingival crest in order to avoid tissue damage. The impression taking and the material mixing techniques were followed according to the manufacturer guidelines. The authors suggest that there isn’t an apparent factor that could identify the gingival recession registered in this study.
The cords soaked in 4% L-epinephrine or in 25% aluminum chloride showed similar gingival displacement capacity when placed in the gingival crevice for 3, 7 and 15 minutes. Additionally, both groups showed significant gingival recession in dogs when they remained in the gingival crevice for a period of 3 minutes or more.
None.
The author declares that there was no conflict of interest.

©2015 Buttendorf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.