Journal of
eISSN: 2373-4396


Review Article Volume 5 Issue 2
Cardiovascular Surgery Department, Salus Hospital GVM Care & Research, Reggio Emilia, Italy
Correspondence: Alberto Albertini, Thoracic and Cardiovascular Department, Salus Hospital GVM Care & Research, Via U Levi 7, 42123 Reggio Emilia, Italy, Tel 390522499111, Fax 390522499162
Received: December 18, 2015 | Published: January 29, 2016
Citation: Albertini A, Caprili L, Amoncelli E. Suture less aortic bio prostheses a reliable tool for minimally invasive AVR in high risk patients. J Cardiol Curr Res. 2016;5(2):119-122. DOI: 10.15406/jccr.2016.05.00154
Background: Surgical aortic valve replacement (SAVR) with extracorporeal circulation (ECC) is currently the treatment of choice for patients with severe aortic stenosis. High-risk patients may benefit from reduced ECC time, cross-clamp time (CCT) and a minimally-invasive approach. Minimally invasive aortic valve replacement (MIAVR) was first described by Cosgrove and Sabik1 and have increasingly gained acceptance in the surgical realm, with the aim of achieving equivalent or superior out- comes compared with conventional AVR (CAVR). A new generation of “suture less” bioprostheses has recently been introduced in clinical practice. Sutureless fixation of the valve combines the advantages of open SAVR, i.e. complete removal of pathological tissue, with reduced ECC and CCT, and facilitates small incision surgery.
Methods: Between November 2012 and October 2015 we operated on 70 consecutive patients with aortic valve stenosis, using suture less valves. Patients (25 males, mean age 77.9±6.3years, range 63-91) had a mean logistic Euro SCORE of 11.9 ± 10.8. Fifty-five patients received a Medtronic 3f Enable and 15 a Sorin Perceval S valve; 48 patients were operated through minithoracotomy, and 22 through ministernotomy.
Results: Valve implantation resulted in a significant improvement in patients’ symptoms; mean preoperative and postoperative transvalvular gradient was 60 mmHg (120-40) and 8.5 mmHg (17-7), respectively. CCT time was66 ± 14.4 min, mean ECC time 71 ± 19.8 min, mean implant time 9 min. 30-day mortality was 1,5%. Early incidence of grade I paravalvular leakages and pacemaker implantation was, respectively, 2.0 % and 4%. 1 late PVL grade 2 was registered.
Conclusion: MIAVR with suture less aortic bioprosthesisin high risk patients represents a safe and effective treatment for aortic valve stenosis, providing excellent hemodynamic and clinical results. A larger study is needed to confirm these initial promising results.
Keywords: aortic valve replacement, sutureless bioprosthesis, TAVI, minimally invasive surgery
SAVR, surgical aortic valve replacement; TAVI, transcatheter valve implantation; PVLs, perivalvular leakages; SU, sutureless; ECC, extracorporeal circulation; CCT, cross clamping time; TEE, transoesophageal echocardiogram; MICS, minimally invasive cardiac surgery; MS, ministernotomy; RAMT, right anterior minithoracotomy; AV, atrioventricular; PM, pacemaker
Surgical aortic valve replacement (SAVR) is the standard treatment for symptomatic aortic valve stenosis. Despite the good results obtained with SAVR, the recent introduction of transcatheter valve implantation (TAVI) is proving effective in the treatment of high-risk patients. But the use of TAVI in younger or mid-risk patients is currently debated, as the incidence of neurological events due to the embolization of calcium fragments, the considerable recurrence of perivalvular leakages (PVLs) and the unknown durability of the valves are still unresolved problems. Nonetheless, the AVR technique can be further improved, and this is the case of the new “suture less” (SU) aortic bio prostheses: these prostheses are designed to allow a fast and simple deployment reducing extracorporeal circulation (ECC) and cross clamping time (CCT) while the absence of sutures makes these valves very versatile in minimally invasive AVR allowing a significant simplification of the procedure. Significant advantages have been reported in terms of postoperative complications, ventilation time and transfusions in patients undergoing minimally invasive AVR.2–6 Only two models of the three SU bioprostheses that were implanted in the last 5 years are currently available in the European Community as the 3f Enable valve (Medtronic Inc., Minneapolis, MN, USA; Figure 1), despite the good results recently published by Englberger et al.7 with a 5-year follow-up, was interrupted this year; the Sorin Perceval S (Sorin, Saluggia, Italy; Figure 1); and the Edwards Intuity Elite (Edwards Inc., Irvine, CA, USA). All SU bioprostheses are basically composed of well-known stentless or stented tissue valves, already widely tested. Even if the initial advantage of using SU valves was indicated as time-saving in ECC and CCT, these valves immediately appeared also very useful for improving the outcome in high-risk patients in minimally invasive AVR. Several series of patients undergoing minimally invasive cardiac surgery (MICS) AVR with SU valves have been published confirming the feasibility of the procedures and with good early results, both for ministernotomy and minithoracotomy.8–10
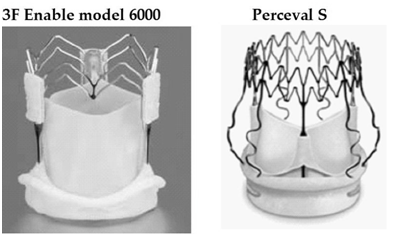
Figure 1 The two collapsible aortic sutureless bioprostheses: Medtronic 3f Enable model 6000 and Sorin Perceval S with the in-aortic root frames.
Patient population and procedures
We began our experience with SU bioprostheses in October 2012 and in the period up to October 2015 a total of 70 consecutive patients [25 males and 45 females, mean age 77.9±6.3years, range 63-91) underwent AVR with SU valves in Cardiac Surgery Departments of GVM Care & Research: Salus Hospital, Reggio Emilia, Italy. Mean preoperative Logistic Euro SCORE 1 was 11.9±10.8. Three patients required urgent surgery for acute heart failure and 4 (5, 7%). Fifty five patients received the Medtronic 3f Enable and 15 the Sorin Perceval S model. Preoperative characteristics of the study population are reported in Table 1. Forty eight (68.6%) patients were operated through a right anterior minithoracotomy (RAMT), 22 (31.4%) through ministernotomy (MS).
|
Variable |
Mean±SD |
Median (min-max) |
|
Age |
79.9±6.3 |
79.5 (65-91) |
|
Logistic EuroSCORE |
11.9±10.8 |
4.74 (0.67-50) |
|
BMI, Kg/m2 |
27.2±4.6 |
26.61 (23.6-30.2) |
|
Preoperative LVEF |
55±3 |
58 (30-65) |
|
Preoperative mean transvalvular |
60±15 |
56 (40-115) |
|
Gradient mm HG |
||
|
Male sex |
25 (35.7%) |
|
|
Hypertension |
45(64.2%) |
|
|
Diabetes |
15 (30.6%) |
|
|
Renal Insufficiency (Scr>2mg/dL) |
6 (8,5%) |
|
|
BPCO |
8(11.4%) |
|
|
Previous PTCA |
5(7,1%) |
|
|
NYHA class II |
22(43,4) |
|
|
NYHA class III |
36(51,4%) |
|
|
NYHA class IV |
12(17,1%) |
|
|
Preoperative AF |
5(7.1%) |
|
|
PM |
|
1(1,4%) |
Table 1 Preoperative characteristics of the study population (n=70)
SD, standard deviation; Euroscore, European system for cardiac operative risk evaluation; BMI, body mass index; LVEF, left ventricular ejection fraction; diabetes: Patients on Medication For Diabetes; BPCO, patients on long-term medication for chronic bronchitis or emphysema and pathologic pulmonary function test; PTCA, Percutaneous transluminal coronary angioplasty; NYHA, New York heart association; AF, atrial fibrillation; PM, pacemaker
As with traditional aortic bioprostheses, implantation requires a cardiopulmonary bypass and aortic cross clamping that are assessed according to the surgical way of access and ascending aorta length. The diseased aortic valve leaflets are removed and the aortic annulus completely decalcified. The sutureless aortic bioprostheses were implanted according to their technique. Intraoperative transoesophageal echocardiogram (TEE) was routinely performed to verify the correct positioning of the valves and to measure the initial hemodynamic parameters.
The hospital mortality was 1, 4%. In 2 (4.08%) patients, a second cross clamp was required due to the intraoperative dislocation of the valve detected by TEE; in both cases the same valve was successfully re-implanted. Mean aortic CCT was 49.6±14.4 min and mean ECC time 62,3±19.8 min. One PVL (grade I) remained at the end of the operation, and it remained stable at discharge and at the 6th month echocardiographic control, in 1 patient a moderate aortic regurgitation occurred three mounts after the operation and the CT scan of the valve revealed a distortion of the prostetic frame causing intra and PVL. Four (5,7%) patients developed atrioventricular (AV) block and needed pacemaker (PM) implant. Stroke occured in 1 patient (1, 4%) and in 2 patients there was a transient disorientation. The mean stay in the intensive care unit (ICU) was 4±4.0days, while the mean overall stay in hospital was 11.3±5.3days.
In our experience with MIAVR using SU bioprostheses the hospital mortality was very low considering the class of risk as the major postoperative complications. The minimally invasive approach to AVR has advantages of decreased ICU and hospital stay, which may be attributed to the reduced surgical trauma. No wound infections occurred in minithoracotomy and ministernotomy but in minithoracotomy, above all, this benefits may be translated from the smaller incision, sternum preservation, and integrity of the costal cartilages. Despite MIAVR has only recently gained acceptance in the surgical community and requires a significant learning curve we found SU valves very easy to implant and we did not have any issue comparing to traditional bioprostheses. We operated patients with isolated severe aortic stenosis with a medium to high operative risk assessed by Euro SCORE 1. Considering our experience in minimally invasive AVR, which in our Departments currently accounts for up to 80% of all AVR, we were mostly interested in implanting SU valves in MICS procedures through RAMT or MS access. CCT and ECC time resulted relatively low but not significantly lower than in AVR performed with conventional bioprostheses. This can be explained considering the learning curve to become confident with the device, in the last quartile of implants performed, in fact, the mean CCT resulted less than 40 min.
In our patient series we analysed only the in-hospital results, a 6-month follow-up is going to be completed only for patients operated through RAMT. Considering the small number of patients, we did not observe any statistical difference in outcome among patients operated with the two different techniques (RAMT, MS). Although we did not compare the clinical data between patients operated with SU valves vs. traditional tissue valves, we found SU valves much easier to implant in MICS AVR than traditional sutured valves; by using SU valves the advantages of MICS could thus be extended to a larger population of patients eligible for AVR.
As previously mentioned, SU valves were initially indicated as offering, in high-risk patients, the benefit of CCT and ECC time savings, but as their use has spread many other benefits have emerged, such as the excellent hemodynamic performance in small aortic annulus and sizes, and the “no need for sutures” has been reported to be crucial in cases of heavily calcified aortic annulus and aortic roots, as in degenerated homo grafts and stent less prostheses. Recently Vola et al.11 reported the first four - successful - cases of totally endoscopic aortic valve replacement: this important innovation opens up a new scenario for possible future applications of SU valves. Initial reports about SU valves are positive and encouraging and our initial experience confirms this, but the limited number of case series published, as well as the lack of prospective randomized trials and long-term follow-up, does not allow any definitive conclusion to be drawn regarding the effective clinical advantages of these bioprostheses. They currently represent an important additional option for special conditions, but as they are demonstrating the same safety, at least in the short- and mid-term, as conventional tissue valves, their use may become a valid option for all patients eligible for biological AVR.
Our experience confirms that sutureless aortic bioprostheses represent a safe and effective treatment for aortic valve stenosis both with traditional and MICS AVR, providing excellent hemodynamic and clinical results. PVL incidence is very low and no prosthesis-related problems were observed. Larger studies are necessary to confirm the clinical benefits of SU valves. A multicenter prospective RCT should be conducted prospectively with adequate power and follow-up duration to measure clinical, resource, and time-related outcomes to definitively assess MIAVR procedures.
None.
The authors declare there is no conflict of interests.
None.

©2016 Albertini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.