Journal of
eISSN: 2473-0831


Research Article Volume 9 Issue 3
1 Center of Maghnia university, Tlemcen, Algeria
2Laboratoire de Chimie Organique, Substances Naturelles et Analyses (COSNA), University of Tlemcen, Algeria
Correspondence: Assia Keniche, Maghnia Center University, COSNA laboratory, Tlemcen University Algeria, Algeria
Received: March 02, 2020 | Published: November 30, 2020
Citation: Keniche A, Taleb KO. Synthesis and simulation of new cyclodextrin derivatives breaker for Aß42. J Anal Pharm Res. 2020;9(3):117-120. DOI: 10.15406/japlr.2020.09.00360
The amyloid Ab-42, a peptide involved, following a conformational change in b sheets in the pathology of the main neurodegenerative disorder of Alzheimer's disease, is targeted in our study, the latter of which reports the synthesis of two Inhibitors linked to a specific recognition sequence synthesized during this work (Tryp-Val-Val-COOH), one linked to an aziridine and the other to a methylated β-CD in order to be able to stop the aggregation of the peptide involved.
Keywords: Alzheimer, peptidomimetic, cyclodextrin, fibrillogenesis
CD, cyclodextrin; Tryp, Tryotophan; Val, Valin
Alzheimer disease (AD) is a neurodegenerative disorder and is associated with accumulation of amyloid and tau depositions in the brain.1–3 If we succeed in blocking fibrillogenesis, we must first block the aggregation of amyloid Ab42, targeting as far as chemists residues responsible for folding. Several studies have been carried out to identify the minimum sequence necessary for the fibrillation of the Aβ peptide. The Aβ19-28 sequence is sufficient to form amyloid fibers.4 As for the first 17 residues of the N-terminal part of the Aβ peptide are not necessary for the formation of amyloid fibers.5 Their elimination makes it possible to amplify the fibrillation. The Aβ16-20 sequence, KLVFF (Figure 1), is designated as necessary for the interaction between Aβ peptides.6,7 Many inhibitors have been developed in recent years, most of which are of peptide nature or are peptide mimics. A large part of the inhibitors is based on the hydrophobic core of the β sheet of the N-terminal domain: the LVFF sequence (Aβ17-20).8–20
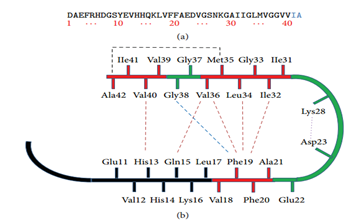
Figure 1 Structure of Aβ40 and Aβ42 that is involved in fibril formation.21
All the reactions with dry solvents were carried out under dry nitrogen. THF was dried over sodium /benzophenone and freshly distilled before use; CH2Cl2 was distilled and dried over phosphorus pentoxide (P2O5). I.R spectra were collected from a Mattson Genesis II FTIR. NMR spectra were recorded in CDCl3 on a Bruker 300MHz instrument, using tetramethylsilane (TMS) as an internal standard. Chemical shifts are given in (ppm) and coupling constant (J) values in Hertz (Hz). ESI-MS data were recorded in the positive ion mode on a quadrupole instrument (Waters-Micromass ZQ). Melting points were determined on an Electrothermal T1A F3.15A instrument. Column chromatography was performed on silica gel 230-270 mesh (Merck) using CH2Cl2, MeOH and ether. Elemental analysis was performed only for solids on a LECO CHN 900 instrument.
Synthesis of b-Sheet breakers A and B
Our approchconsist todevelopp-sheet breaker peptides. Several derivatives peptides have been synthesized. The amyloid deposition could be stopped by synthetic peptides partially homologous to the Ahydrophobic region and containing residues disrupting -sheet formation (Figure 2). Our synthetic pathways to our target aziridines 6a-e are presented in Schemes 1 and 2. The synthesis of tosylate aziridine 4, was achieved by the O-protection of glycidol 1 with p-toluenesulfonyl chloride (TsCl). And was treated with ammonium chloride and sodium azide to give the azido alcohol 3, which was reacted in the next step with a solution of triphenylphosphine (PPh3). The biological activity of aziridines is highly related to the establishment of covalent bond with DNA.22 In a previous investigation of our group we noted the synthesis of aziridinyl that had antitumor activities against breast cancer cells (Figure 3).23,34
We have, already reported the synthesis of several aziridines,25–27 that moiety of amino acids phtaloyl group with a phosphonate, surprisingly, the biological activity of seri of phosphonates aziridines shifted from antiviral to an antibacterial one.28–31 For the second inhibitor, we choose b-cyclodextrine (-CD) as disrupting group coupled with the same specific recognition sequence of tripeptid (Try-Val-Val) (Figure 4).
Cyclodextrins (CDs) are compounds that are produced by the enzymatic degradation of starch. The three most common CDs have 6, 7, and 8 glucopyranose units in the cyclic and are named α-CD, β-CD, and γ-CD, respectively.32 Whereas the depth of the cavity for these CDs is∼8 Å, the sizes of the cavity are different for α-CD, β-CD, and γ-CD, being∼6, 8, and 10 Å, respectively (Figure 5).33–44
So our idea to incorporate a cyclodextrine for improvement of vectoraziation of tripeptid in vivo and to insert a large disturbing group between the b-sheet of Aβ agragation which will imply the rupture of the hydrogen bonds. In addition, the intra-inclusion study of chain Try-Val-Val or inclusion of (Aβ-42) inside the cavity of CD can improve the interaction and blocked the Amyloid Fibrillogenesis.
Tripeptid Try-Val-Val 5 was reacted either with thionyl chloride in the presence of TEA to yield, an acyl chloride that was reacted with aziridine 4 to give breakers A, or coupled in the presence of dicyclohexylcarbodiimide (DCC) as coupling agent with CD at room temperature, giving the breakers B in medium yield (50-65%) (Figure 6).
Primary result of study of interaction of breakers A with A42
The study by MOE docking showed that structure of brekears A with only aziridine moiety group was the best interaction than B with A42 (Figure 7). Only biological evaluation in vivo can give wish are the best inhibitors (Figure 8).
To conclude we can say that eradicating Alzheimer's disease in a radical way, we are not yet there but the fact to look into this problem by studying the issue of beta amyloid (Aβ-42) remains a fairly studious approach. We developed two inhibitors A and B with aziridine or CD as disrupting group to be able to stop the fibrillar aggregation, a way still remains to be done to really develop them. in order to ensure their effectiveness, it is necessary to confirm their biological activity. At least in terms of synthesis, the method followed is simple; conventional peptide couplings, which can be improved.
The authors are grateful to Ministry of Higher Education and Scientific Research (Algeria) for the financial support of this work.
The author declares that there are no conflicts of interest.

©2020 Keniche, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.