Journal of
eISSN: 2473-0831


Review Article Volume 4 Issue 3
Correspondence: Yakubu OE, Department of Biochemistry, Faculty of Pure and Applied Sciences, Federal University Wukari, Nigeria
Received: March 01, 2017 | Published: March 8, 2017
Citation: Wong WKR, Ng KL, Lam CC, Hu XH, Lai NCY, et al. (2017) Review Article: Reasons for Underrating the Potential of Human Epidermal Growth Factor in Medical Applications. J Anal Pharm Res 4(2): 00101. DOI: 10.15406/japlr.2017.04.00101
Human epidermal growth factor (EGF) is a functionally versatile polypeptide comprising 53 amino acids (aa). Due to the ability of EGF to stimulate epidermal growth and proliferation, numerous research studies have been conducted to explore its potential applications in cosmetic, skin care and medication. The emergence of recombinant DNA technology has facilitated the production of a great variety of recombinant EGF (rEGF) isoforms. However, increasing evidence supports that rEGF isoforms exhibit different levels of stability and potency. This review discusses how the less bioactive rEGF isoforms may mystify the identity and even the functional properties of authentic rEGF, which shares the same primary structure as native EGF. The identity crisis, the time- consuming, costly and labor-intensive patenting and drug regulatory processes act together to result in underrating the functional applications of authentic rEGF, which have been shown to be effective and safe in promoting treatments of a variety of skin disorders such as diabetic foot ulcers (DFU).
Keywords EGF, Authentic, Hard-to-heal wounds, Topical medication, Derivatives, Recombinant, Murine, Eruption, Treatments, Physiological, Chronic wounds, Healing,
Degradation
EGF, human epidermal growth factor; rEGF, recombinant human epidermal growth factor; aa, amino acid(s); dfu: diabetic foot ulcers
Human epidermal growth factor (EGF) was first found to be present in a pregnant woman’s urine sample in the mid-1970s.1 Prior to this discovery, mouse EGF was first isolated and shown to promote incisor eruption and eyelid opening in murine neonates in 1962.2 Since then EGF was shown to exhibit a variety of physiological functions in animals such as healing of epidermal wounds,3 corneal injuries4 and burns.5 Recently, with human subjects, EGF has been shown to be effective in promoting treatments of both acute and chronic wounds, including scalds, Stevens-Johnson syndrome, diabetic foot ulcers and bedsores.6 Moreover, EGF has also been exploited for enhancing skin tone and texture in cosmetic industry in the last two decades.7
The functional versatility and high marketing prices of EGF, averaging US$ 2,000permg,8-10 have provided investors with a strong incentive to bring forth new protocols, hoping to produce EGF cost-effectively. The emergence of recombinant DNA technology has facilitated the evolvement of a wide variety of strategies and expression systems, among which many have been employed for recombinant EGF (rEGF) expression.
Due to the concern that the small (53 aa long) EGF polypeptide might be susceptible to proteolytic degradation, strategies involving the use of fusion protein partners have been commonly exploited for use in expressing rEGF (Table 1). A major drawback of the fusion approach is the formation of rEGF as variants possessing different peptide lengths (Table 1).11-22 Moreover, the EGF derivatives were commonly shown to exhibit lower levels of bioactivity and stability.17,22-24 Later on, pragmatic approaches were developed for the production of not only bioactive and stable rEGF, but also a product available at a much reduced price.11-12
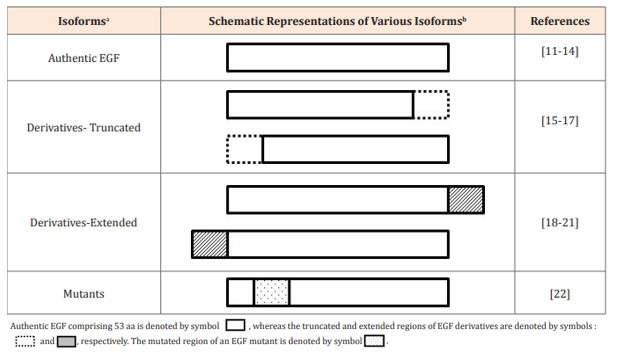
Table 1 Authentic EGF and its structural derivatives
aDifferent EGF isoforms include authentic EGF comprising 53 aa, its truncated (less than 53 aa) and extended (more than 53 aa) derivatives, and mutants (with different aa compositions).
bSchematic representations of various EGF isoforms .
Disparities in Performance among Different rEGF Isoforms
The rEGF products available from various expression activities were analyzed for their efficacies in promoting treatments of skin diseases, including hard-to-heal wounds such as diabetic foot ulcers,25-28 bedsores,29 scalds30 and Stevens-Johnson syndrome,31 of which the last disorder results usually from a severe allergic reaction to a medication.32-33 Despite the positive outcomes shown in these studies, the efficacies of EGF as an active ingredient in skin care or medical applications have been controversial, in particular in studies conducted in earlier times.34
One possible reason for the inconsistent results is the use of structurally different EGF molecules, notwithstanding that all samples involved are indiscriminately designated “EGF” and very often, the quality and exact identity of EGF concerned were not revealed in these studies, thus restricting the audience to assume that an active EGF product was employed in the work.35-38 However, more and more findings support that authentic EGF (of which the primary sequence, comprising 53 aa, is the same as that of native EGF), is much more potent and stable than its unauthentic counterparts.
The variability of efficiencies exhibited between authentic EGF and its structural analogs in functional performance is likely, on the one hand, attributable to the unstable nature of the derivatives, which have been reported to be susceptible to proteolytic degradation.17 On the other hand, the exact structural composition and purity of a recombinant product could also affect its performance. Research findings strongly support that authentic rEGF with high purity is remarkably stable, despite its maintenance and usage in the absence of protein stabilizers.12 In addition, among the great variety of rEGF isoforms reported in the literature, so far, only the authentic form has been shown to exhibit high efficacies, despite through simply topical administration, in promoting wound healing.30 The shorter derivatives comprising 51 and 52 a residues have been shown to be less active and their application to treatments of DFU has to be done through intralesional administration.39 These discrepancies in performance among different rEGF isoforms, which are all collectively called “rEGF”, have confused the outcomes of the mentioned studies.
More controversial issues concerning rEGF applications
Taking advantage of the lenient approval and monitoring activities in cosmetic and skin care industries, rEGF isoforms have been applied commercially to result in a wide collection of marketable end products, irrespective of the absence of documentation of their origins and structural properties.40-41 Obviously and understandably, the feedbacks received from the customers or end users, have been highly controversial.
Since EGF is responsible for growth of the epidermis, there is often some speculation as to whether EGF is carcinogenic. Recently, there have been numerous publications reporting the expression and applications of rEGF. Despite the rapid gathering of evidence supporting the wide application of rEGF to skin care and treatments of a wide range of skin problems,6,30 interestingly, there has not been any concrete evidence to support that the polypeptide could trigger carcinogenesis.42 Apparently, authentic rEGF is highly safe, at least, for use in topical administration. In fact, the harmless nature of EGF has been well documented by Dr. Cohen in his pioneering work with murine EGF in the early 1960s.2,43 However, the concern regarding whether unauthentic rEGF molecules are as safe as authentic rEGF upon administration requires further study to clarify.
Additional Hurdles for rEGF to Overcome on its Path to Medicine
Given the demonstration that authentic rEGF is effective in treating a variety of human wounds through topical administration,6,26,30-31 and that the protocol employed is simple and safe,6,26 if a license is sought for rEGF to be applied as a drug in a country, a lengthy and expensive process, which includes:
The total time required to complete all these three undertakings can easily be exceeding 10 years.
The application of rEGF in enhancing treatment of DFU employing simply a topical approach has been well documented in many separate and independent studies.26,29 Presumably, quality authentic rEGF was employed in these studies; the success rate of complete healing of DFU increased from less than 50% found in placebo controls, wherein only debridement was administered,26 to as high as 95% observed in patients treated with both debridement and rEGF.26 This facile and effective treatment protocol,26 although it is not yet officially approved for medical applications, has been employed successfully to treat thousands of DFU (unpublished data). Intriguingly and obviously, the protocol is very safe as there has not been a single incident of side effect observed in individuals treated by the topical method described.
Years of devoted research have resulted in cost-effective protocols for authentic rEGF production, and substantial evidence to support the effectiveness of the simple and safe topical protocol in enhancing treatments of DFU.26 Paradoxically then, the long durations taken for patent application and FDA clearance and approval processes, which are meant to benefit the inventors/investors and patients, respectively, deprive the opportunities of millions of DFU patients from enjoying the prompt healing effect of rEGF simply through topical treatment, and more importantly, from avoiding amputation, which will result in devastating consequences. Moreover, the potential beneficial effects of the topical medication protocol on the treatments of DFU and other kinds of hard-to-heal wounds, e.g. bedsores29 and surgical injuries,6 are expected to help substantially reduce the enormous medical expenses incurred for treatments of these skin problems.
Recombinant human epidermal growth factor (rEGF) possessing the native 53 aa primary structure has been shown to be effective and safe in enhancing treatments of various kinds of skin problems including hard-to-heal wounds such as diabetic foot ulcers and bedsores, simply through topical administration. However, the availability of varieties of rEGF derivatives, which are also commonly called “rEGF or EGF”, has mystified the identity and functional properties of authentic rEGF. The confusions have imposed a negative impact on the credibility and applicability of authentic rEGF. Moreover, its potential for drug applications is further weakened by the time-consuming, costly and labor-intensive patenting and drug regulatory processes.
This study was partially funded by RGC project: GRF16101515 awarded to W.K.R. Wong.
The authors do not have any personal or financial interests.
None.

©2017 Wong, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.