Journal of
eISSN: 2473-0831


Research Article Volume 3 Issue 1
Correspondence: Wong WKR, Division of Life Science, Hong Kong University of Science and Technology Clear Water Bay, Kowloon, Hong Kong, Tel (852)23587299, Fax (852)23587299
Received: August 05, 2016 | Published: September 1, 2016
Citation: WKR W, Kwong KWY, KL NG (2016) Application of Recombinant Human Epidermal Growth Factor to Effective Treatment of Scalds. J Anal Pharm Res 3(1): 00045. DOI: 10.15406/japlr.2016.03.00045
Burns and scalds are commonly encountered incidents among the household accidents. Patients suffering from second-degree scalds may encounter further complications of blister formation, infections, prolonged healing and even scarring if the scald wounds did not receive proper treatment initially. With authentic recombinant human epidermal growth factor (rEGF) expressed in our laboratory, we report in this communication that rEGF, available at a final concentration of 0.04% (w/w), was highly effective in treating three cases of second-degree scald injuries. Despite encountering delayed treatment with rEGF, the scald wounds were shown to experience effective healing, in which exfoliation of dead skin and regeneration of epidermal cells without scarring were enhanced. Moreover, rEGF was also shown to be effective in treating freshly scald skin, thus helping the affected area to relieve pain and to prevent blister formation.
Keywords: scalds, EGF; bFGF, blister, hard-to-heal wounds, authentic primary structure, biological activity, burns
EGF, human epidermal growth factor; bFGF, human basic fibroblast growth factor; EC, enhanced cream; SGP, skin growth proteins; rEGF, recombinant human epidermal growth factor
Residents of a developed society enjoy various kinds of new residential, commercial and industrial developments. Many of these developments, however, may get us exposed potentially to hazardous accidents upon applications. Among them, burns and scalds are probably the most commonly encountered incidents.1–3 For example, electric appliances like sprinklers, kettles, hot pot cookers, steam/dry irons, etc., may produce overheating contact point(s), liquid or vapor that causes damages to the skin on various parts of our body. Among patients of scald injuries, babies are the most common victims and hot drinks are the leading cause of scalds.1
Scald victims experience tissue damage to the area of the wound. Depending on the depth of skin being affected, different degrees of burn are classified. A superficial burn such as sunburn that affects the epidermal layer of skin is classified as a first-degree burn. It is usually characterized by mild pain, redness and peeling. A second-degree burn is classified and characterized by additional pain sensation and blister formation on the skin. A third-degree burn suffers an even deeper damage involving all layers of skin; moreover, the nerves and blood vessels are also damaged. The skin will turn white and lose sensation.2
First-aid treatment for scalds involves stopping the burning process as soon as possible, followed by applying cool running water to cool down the skin to help reduce damage immediately.3 In addition, the wound is left uncovered and moisturized periodically with a non-scented, non-greasy moisturizer.4 When blisters form on an injured area, although sometimes deroofing a blister is necessary, it is recommended not to burst a blister as it may increase chances of infection. For small blisters with pale pink or mottled appearance, they may take 3-8 weeks to heal without or with minimal scarring. However, regarding deep dermal scald injuries forming large botchy cherry red blisters, it may take over 8 weeks or a prolonged time to heal with potential scarring,5 and may require surgical treatments for functional recovery.5
Our laboratory has been involved in the development of microbial systems for cost-effective production of useful or valuable recombinant proteins.6–14 Among the target proteins, we have been interested in skin growth proteins, including human epidermal growth factor (EGF) and human basic fibroblast growth factor (bFGF), which are key members among skin growth proteins in providing maintenance and regeneration to the epidermal and dermal layers, respectively, of our skin.
Both EGF and bFGF have been discovered over 30 to 40 year.15–17 During this long period of time, both factors have been studied extensively and shown that they are involved in a wide range of biological functions,15,17–24 supporting that they are potentially applicable for use as therapeutics for treating various physiological disorders. However, both EGF and bFGF cost hundreds of thousands of USD for just one gram of product, despite the fact that both factors have been shown to be highly active in promoting wound healing18,20,23,24 and growth of various cell types.25,26 The high prices of EGF and bFGF restrict their research for clinical applications to certain wound types and small-scale studies.
Employing efficient bacterial systems developed in our laboratory, we have been successful in cost-effective production of recombinant EGF and bFGF possessing authentic primary structures.8,9,11 Results from recent studies have offered support to the view that sequence authenticity is not only important for recombinant EGF and bFGF to maintain their structural stability,8,9,11 but is also crucial for both factors to acquire potent bioactivities.8,9,22,24 Previously, we have made use of cream base supplemented with 0.04% (w/w)of our recombinant EGF (rEGF) to successfully treat sporadic cases of scald injury, with an example shown in Figure 1. However, the healing process of these injuries was not well monitored. In this communication, we report, with careful monitoring of the progress of recovery, the use of rEGF for effective treatment of three incidents of scald injury, even in cases where blisters had burst and red patches of skin had emerged.
Source of recombinant EGF
Expression of recombinant EGF (rEGF) was achieved using Escherichia coli JM101 [pWKW2] transformant,11–13 in which the conditions for induced production of rEGF were described previously.8,11–14 Details for purification of rEGF, characterization of the authentic structure of rEGF, and confirmation of rEGF being devoid of either toxic or allergenic property have been reported previously.8,11
Preparation of treatment cream containing rEGF
The cream base, named Watsons Aqueous Cream27 was purchased from Watsons Hong Kong. To prepare the treatment cream containing rEGF, purified rEGF dissolved in sterile ddH20 was added into the cream base to a final concentration of 0.04% (w/w). The content was then mixed thoroughly until rEGF was evenly distributed to form the final product, designated “Enhanced Cream” (EC), with rEGF supplemented to a final concentration of 0.04% (w/w).
Treatment of scalds with EC
In treating a burst or broken blister resulting from a previously scalded wound, the affected area was first cleansed thoroughly with diluted dettol23 as recommended by the manufacturer. The disinfected area was then topically applied with a slight film of EC, followed by covering with a gauze pad as described previously.23 The application was done twice a day.
With respect to a fresh scald injury caused by hot water, EC was applied swiftly to the affected area. If the scald results from hot fluids other than water, it is advisable that the affected area is first rinsed with cool water, then dried with a clean tissue or soft towel, followed by applying EC to the affected area.
Treatments of previously scalded wounds
Treatments of three female victims of different ages suffering scald injuries in different parts of the body caused by hot water were reported. In all three cases, the scalds failed to receive immediate medical care, thus leading to the formation burst blisters, and subsequently to the appearance of red to cherry red patches on skin.
Case 1: A 1-year-old toddler girl suffered second-degree scalding burns on her submental region (Figure 2) in Guangzhou, China. Soon after the accident, a large painful blister was developed on the girl’s scalded chin. She was attended by a medical doctor in a clinic and was prescribed a cream supplemented with a locally produced skin growth factor (personal communication with the girl’s father) for topical application onto the wound. Despite being treated with the cream and visiting the clinic twice, the girl still groaned in pain and the blister subsequently burst; there was no sign of improvement (Figure 2). The parents were even warned that despite recovery, the scalding injury might still leave a scar on the girl’s chin.
The girl’s parents were then referred to us by a friend who knows our accomplishments in treating ulcers and other wounds with recombinant human epidermal growth factor (rEGF).22–24 Immediately, we prepared “Enhanced Cream” (EC), which contained 0.04% (w/w) rEGF (Materials and methods), for treating the girl’s scald injury, which began on the 4th day after the accident.
On the 1st to 5th day of the treatment, the wound showed peeling of remnants of the burst blister (Figure 2). In addition, there appeared healing signs as reflected by: signification pain reduction in the girl’s wound, smoothening of the boundaries of the original scald, and discoloration of the pinkish red patch on the skin. In the next nine days, the wound healed readily and complete healing appeared to occur in two weeks without scarring (Figure 2).
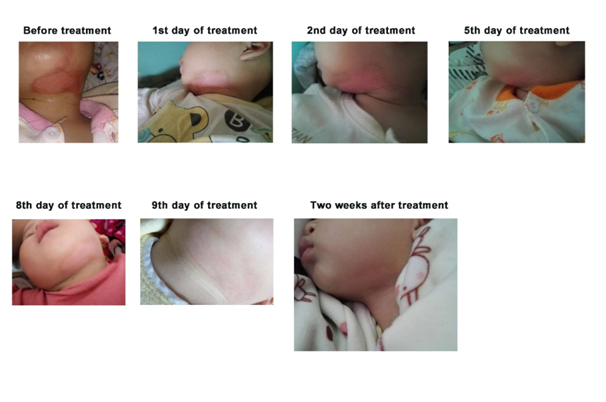
Figure 2 Treatment of an unhealed scalding wound on the submental region of a toddler girl with EC supplemented with 0.04% (w/w) rEGF. The duration in day(s) of the treatment is shown on top of the picture. Before treatment stands for before treating with EC.
Case 2: Concurrently, similar to the first case, a 7-year-old girl residing in Hong Kong also suffered second-degree scalding injuries; but this time, the affected region concentrated essentially on the left thigh (Figure 3). Blisters formed swiftly on the scaled skin and subsequently burst. The girl was sent to see a clinician on the next day after the accident and Silvirsulf Cream28 was prescribed for treating the scalds. Soon after the treatment, the girl’s father reckoned that the cream might not have a significantly positive effect on healing the wounds; he then approached us and procured EC to treat his daughter’s injuries. EC was applied to the wounds as mentioned in Materials and methods. The medical doctor initially predicted that it would take at least two weeks for the wounds to heal. However, after 12 days of EC treatment, it was quite surprising to observe that the wounds showed apparently complete healing (Figure 3). Subsequently, on the 14th day of EC treatment, the wounds archived complete healing without scar formation.
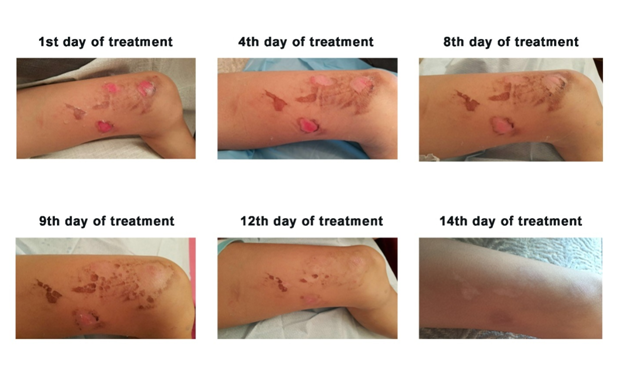
Figure 3 Treatment of scalding injuries on the thigh region of a 7-year-old girl with EC supplemented with 0.04% (w/w) rEGF. The duration in day(s) of the treatment is shown on top of the picture.
Case 3: In the third case, a 30-year-old female Hong Kong citizen suffered scald injuries resulting from steam generator bought from China while she was employing the machine to facilitate foot massage treatment. However, while the massage was in process, the machine suddenly exploded. Since it was quite an explosion, the lady was much more seriously scalded than the two patients referenced in the first and second cases. Certain parts of the scalds were considered as severe second-degree to even third-degree burns (Figure 4). She was admitted to St. Teresa’s Hospital and was subscribed a 2.5% sodium hyaluronate based wound dressing29 for the management of the wounds. Despite treatment with the dressing for 11 days, there was only little improvement in the healing of the wounds. The patient then switched to the use of EC to replace the dressing to provide continuous treatment of the scalds. Despite delayed treatment with EC, the progress of healing of the wounds was phenomenal. With only one day of treatment, discoloration of the reddish patches and regeneration of new skin were significantly noted (Figure 4). After six days of treatment, the wounds were apparently healed without any signs of formation of the previously concerned side effect – scarring (Figure 4).
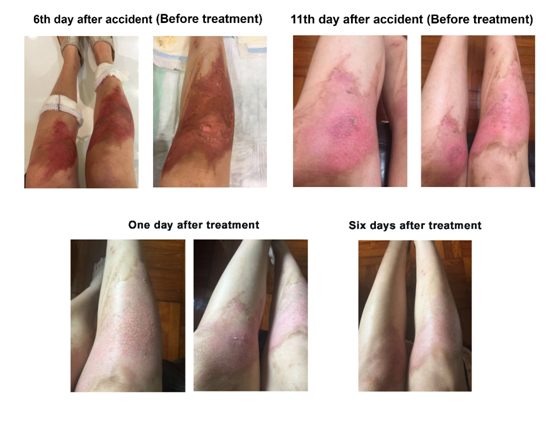
Figure 4 Treatment of scalding injuries on the two legs of a 30-year-old woman with EC supplemented with 0.04% (w/w) rEGF. The duration in day(s) of the treatment is shown on top of the picture. Before treatment stands for before treating with EC.
Treatment of freshly scalded skin
An effective way of treating freshly scalded injuries caused commonly by spilled hot liquids, e.g. water, may have to avoid subsequent complications including formation of blisters, potential scarring5,30 and requirement of surgical treatments for functional recovery.5 In an accident, a 30-year-old male got scalded by boiling water spilled on his right forearm (Figure 5A). The scald was immediately treated with EC, of which an excessive amount was applied to fully cover the wound. The cream was then left uncovered and the application lasted for at least one hour. Upon application, EC provided the patient with instant relief from pain. Moreover, after one hour of EC application, there was no sign of blister formation or change of color of the skin where the scald wound was initially noted (Figure 5A). Even without further treatment with EC, the forearm appeared to be normal and showed no scalding sign (Figure 5A).
Notwithstanding the effective performance of EC in protecting the scalded skin from further development of harmful side effects including blister formation (Figure 5A), an appropriate administration of EC to a scald wound appeared to be crucial to effective application of EC. For example, the duration of EC application to a scald injury was too short, as it was observed in treating the hot water spill on the 62-year-old male’s forearm (Figure 5B). Although EC was applied to the wound right after the accident, in only 15 min or so, the treated area was covered with a bandage until the next morning. This format of EC application appeared to be still effective in reducing pain (personal communication). However, quite unexpectedly, despite the treatment, a fluid-filled blister formed on the scalded skin (Figure 5B).
There have been a number of articles reporting the beneficial effect of EGF in healing of scald wounds in both animal and human subjects.31–34 Notwithstanding the supporting data shown by these reports, EGF has still been scarcely employed as remedy for treating scald or burn injuries. The concerns about safety, high prices and inconsistent qualities of EGF have presented as major obstacles to the widespread application of EGF to the treatment of burn and scald wounds. These difficulties have been related to cost-effective production of quality EGF for commercial applications.
Our laboratory has endeavored to develop efficient microbial systems for large-scale production of recombinant EGF (rEGF).8,11-14,22–24 The resulting rEGF is not only available at a much lower price, which is about 10-15% of that of its commercial counterparts,35 but is also verified to possess an authentic structure of 53 amino acids and free from toxic and allergenic activities.11
Using rEGF produced by our group and other teams,10,13,36 we have demonstrated that rEGF is effective in treating a number of skin wounds including diabetic foot ulcers,24 Stevens-Johnson syndrome22 and surgical wounds.23 Despite the well-defined efficacies of rEGF revealed in treating various wounds and other healthcare applications,22–24 rEGF available from other sources might not be able to yield the same or reproducible results as ours, thus creating confusion and uncertainty regarding the exact effectiveness of rEGF in applications. In many of these studies, however, the chemical structure and quality of rEGF employed have not been discretely specified. It has been shown that skin growth factors possessing unauthentic structures are either highly susceptible to degradation or biologically less active.37 Thus, using EGF with compositions different from that of native EGF in experimental work could seriously affect the overall research outcome. In this regard, there have been reports claiming that EGF was ineffective in treating scald or burn injuries.33 These controversial results, unfortunately, would reduce our confidence in further development of rEGF for drug applications, which have been shown to be potentially broad.22–24,33,34
In view that unexpected patients receiving immediate medical treatments were the best witnesses regarding whether hEGF was effective in treating scald wounds, EC was freshly prepared and employed to treat victims suffering previously or freshly scald injuries caused by hot water spillage. It was apparent that continuous application of the commercial products, e.g. Silvirsulf Cream (see Case 2 in Results;28) would not result in more beneficial outcomes in the three cases of previously scalded wounds. Further delay in treating the wounds with effective medications could lead to complications including infections and scar formation after healing. Obviously, with application of EC to the wounds, the healing process was effectively speeded up. In all three cases, the processes of shedding dead skin and regeneration of new skin were enhanced (Figures 2–4). Last but not least, the wounds healed well without scar formation (Figures 2–4).
We were surprised to note that prolonged application of EC to a freshly scalded wound was effective in not only alleviating pain, but also preventing blister formation (Figure 5A). It has been postulated that blister formation begins with epidermal cells in the prickle-cell layer that have lost their structural connections. This area of separation of cells will then be filled with fluid within 2 h to form a blister.38 Although the exact mechanism of how EC functions to prevent blister formation, presumably it works to maintain integrity of the epidermis, in particular the prickle-cell layer, thus avoiding subsequent influx of fluid to form the vesicle, in not known. In order to keep the cell layers intact, it might be necessary for rEGF to be kept on the scalded skin for a long enough time to exert its function. The interpretation might help to explain why the scalded wound, which was covered with a bandage soon after EC was added for only 15min, on the 62-year-old victim resulted in the formation of a blister (Figure 5B). Therefore, the presence of the bandage might, on the one hand, “take away” by absorbing some of the applied EC, and on the other hand, affect oxygen supply, which might be required by rEGF for functional activity. This postulation gains support from previous findings that sufficient transport of oxygen and nutrients to injured tissues is important to allow scalded areas to achieve better skin regeneration and recovery.39
Regardless the exact mechanism of how rEGF facilitates healing of scald wounds, our results suggest that rEGF may serve an effective first-aid treatment for a scald or burn injury, which may further be developed to harmful side effects including blister formation, infections and scarring upon healing if the wound was not effectively treated initially. Since we have employed a high concentration, 0.04% (w/w), of rEGF in all the treatments discussed, it may be economically worthwhile to see whether there exists a dose-response relationship between rEGF concentration and effective treatment of different severities of scald or burn injuries.
Authentic rEGF containing 53 amino acid residues is effective in treating scald injuries. Aqueous cream containing 0.04% (w/w) rEGF (EC) was shown to be able to promote regeneration and restoration of second-degree scald wounds, previously or freshly incurred. On previously scalded skin, EC helped to exfoliate dead skin, promote epidermal cell growth, and prevent scar formation. While on freshly scalded skin, prolonged EC treatment could prevent blister formation and development of other complications.
This study was funded by RGC project: GRF16101515 and Research Contract: 13142580CLIL07W011 awarded to W.K.R. Wong.
The authors declare there is no conflict of interests.
None.

©2016 WKR, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.