Journal of
eISSN: 2378-3184


Research Article Volume 6 Issue 1
Department of Zoology, North-Eastern Hill University, India
Correspondence: BK Sharma, Freshwater Biology, Department of Zoology, North-Eastern Hill University, India
Received: June 12, 2017 | Published: July 18, 2017
Citation: Sharma BK, Hatimuria MK (2017) Zooplankton Diversity of Three Floodplain Lakes (Beels) of the Majuli River Island, Brahmaputra River Basin of Assam, Northeast India. J Aquac Mar Biol 6(1): 00144 DOI: 10.15406/jamb.2017.06.00144
The biodiverse zooplankton of Bhereki, Holmari and Ghotonga beels of Majuli River Island, the Brahmaputra river basin of upper Assam, northeast India (NEI) revealed total richness of 141 (118±8) species and thus suggested habitat diversity of these floodplain wetlands. Low community similarities, monthly richness variations and the cluster groupings affirmed heterogeneity of zooplankton species composition. Zooplankton formed the dominant component of net plankton in Ghotonga beel and showed sub-dominance in Bhereki and Holmari beels. Rotifera > Rhizopoda influenced zooplankton density in Bhereki and Ghotonga beels; Rhizopoda > Rotifera showed importance in Holmari beel; and Copepoda > Cladocera recorded sub-dominance in all beels. Zooplankton is characterized by higher species diversity and equitability, and lower dominance. The richness, abundance and diversity of zooplankton and abundance of the constituent groups followed oscillating monthly variations. While explaining limited influence of individual abiotic factors and low cumulative influence along two axes (vide Canonical Correspondence Analysis), our results suggested that zooplankton are largely generalists in terms of abiotic factors and thus hypothesized importance of factors associated with microhabitat.
Keywords: Aluvial floodplains, Abundance, Composition, Density, Richness, Tropical Wetlands
The floodplain lakes form an integral component of various riverine systems globally and merit interest for biodiversity and ecology considerations. These remarks hold valid for the Indian floodplains and those of northeast India (NEI) in particular.1-3 These wetlands form a lucrative source of inland fishery4 of NEI and are locally known as ‘beels’ in Assam and pats in Manipur. Further, zooplankton contribute importantly to metazoan diversity and production of fish-food organisms in the wetlands but have yet received inadequate attention on their diversity and ecology in the floodplain lakes of India1 while the related published ecology works from NEI are limited to reports from certain beels of Assam5-7 and pats of Manipur.8,9
Majuli, a geologically interesting landform of fluvial geomorphology of the Brahmaputra river basin of upper Assam as well as a world heritage site, is under threat of extinction because of alarming erosion. This largest riverine island is literally dotted with beels with vital socio-economic importance due to notable fisheries potential. Our knowledge of plankton communities in the floodplains of Majuli River Island is limited to the faunal diversity of Rotifera10,11 and Cladocera.12 This first study on zooplankton diversity of selected Majuli beels merits ecological and aquaculture interest in the Indian floodplain lakes in general and those of NEI in particular; referring to the latter aspect, these floodplains presently yield 500-700 kg fish/ha/yr which can be significantly enhanced 3-4 times through scientific management4 based on knowledge of diversity and production of fish-food organisms. The observations are made on monthly variations of richness and abundance of zooplankton and its constituent groups vis-a-vis individual and cumulative influence of abiotic factors; and community similarities, species diversity and evenness and dominance.
This limnological survey was undertaken during September, 2010–August, 2012 in Bhereki beel (94o08′23.3″ E, 26o55′40.4″ N; Altitude: 72 m ASL), Holmari beel (94o12′30.6″E; 26o59′17.3″N) and Ghotonga beel (94o15′28.7″E, 27o01′52.7″N; Altitude: 69 m ASL) located in Majuli River Island in the Jorhat district of Upper Assam (N. E. India). The sampled wetlands indicated a diversity of macrophytes namely Eichhornia crassipes, Hydrilla verticellata, Utricularia flexuosa, Trapa bispinosa, Lemna major, L. minor, Pistia striates, Salvinia sp., Nymphaea spp., Nymphoides spp., Vallisneria spiralis, Euryale ferox, Xanthium sp., Ipomoea fistulosa & Sagittaria sp.
Water samples were collected at regular monthly intervals and analyzed for various abiotic factors. Water temperature, specific conductivity and pH were recorded by field probes, dissolved oxygen was estimated by the modified Winkler’s method and other parameters were analyzed following APHA.13 Qualitative zooplankton samples were collected from the floodplain lakes by towing any lobolt plankton net (# 50 µm) and preserved in 5% formalin. These samples were subsequently screened for various zooplankton species and their permanent mounts were made in polyvinyl alcohol-Lactophenol mixture.
Monthly quantitative zooplankton samples were also obtained by filtering 25 litres of the lake water through nylobolt plankton net (No. 25). Individual collections were then concentrated to 25 ml each and preserved in 5% formalin. The quantitative enumeration (n/l) was done with the help of a Sedgewick-Rafter counting cell. The zooplankton was identified following the works of.1,14-19 Quantitative samples were analyzed for abundance of zooplankton. Community similarity (Sørensen’s index) and species diversity (Shannon’s index) were calculated following.20,21 ANOVA was used to analyze the significance of temporal variation of the biotic communities. Ecological relationships between abiotic and biotic parameters of Bhereki beel, Holmari beel and Ghotonga beel were determined by simple correlation co-efficient (r1,r2 and r3, respectively); P values were calculated and their significance was ascertained after the use of Bonferroni correction. The canonical correspondence analysis (XLSTAT 2014) was done to analyse cumulative influence of seventeen abiotic parameters (water temperature, rainfall, pH, specific conductivity, dissolved oxygen, free CO2, total alkalinity, total hardness, calcium, magnesium, chloride, dissolved organic matter, total dissolved solids, phosphate, nitrate, sulphate and silicate) on the zooplankton assemblages.
The ranges and mean ± SD of the recorded abiotic parameters of Bhereki, Holmari and Ghotonga beels are indicated in Table 1 and of different aspects of zooplankton diversity are included in Table 2. We observed a total of 141 with 111, 113 and 129 species in three beels, respectively (Table 2). Rotifera is represented by 70, 66 and 79 species in Bhereki, Holmari and Ghotonga beels, respectively (Table 3) and indicated qualitative importance of Lecanidae > Lepadellidae > Brachionidae. The monthly zooplankton richness varied between 45–64, 47-67 and 49-76 species (Figures 1-3); it recorded 48.8–78.3%, 49.1–74.3% and 48.7–69.7% community similarities (vide Sørensen’s index) in three beels, respectively. The hierarchical cluster analysis of zooplankton is presented in Figures 4-9. The monthly rotifer richness ranged between 21–35, 22–39 and 23–45 species while that of Cladocera (33 species) varied between 5–14, 4–14 and 8–18 species the sampled beels, respectively.
|
Factors |
Bhereki beel |
Holmari beel |
Ghotonga beel |
|||
|
Range |
Mean ± Sd |
Range |
Mean ± Sd |
Range |
Mean ± Sd |
|
|
Water temperature (oC) |
21.5 – 27.5 |
23.7 ± 1.7 |
21.0 – 27.5 |
23.6 ± 1.7 |
21.5 – 27.5 |
23.9 ± 1.7 |
|
Rainfall (mm) |
0.0 – 413.76 |
142.57± 133.90 |
0.0 – 413.76 |
142.57± 133.90 |
0.0 – 413.76 |
142.57 ± 133.90 |
|
pH |
6.29 – 7.41 |
6.67 ± 0.23 |
6.56 – 7.13 |
6.87 ± 0.13 |
6.17 – 6.85 |
6.51 ± 0.16 |
|
Conductivity(µS/cm) |
102.0 – 189.0 |
140.7 ± 24.4 |
111.0 – 220.0 |
173.6 ± 32.5 |
73.0 – 182.0 |
121.4 ± 26.8 |
|
Dissolbed oxygen (mg/l) |
4.8 – 8.0 |
6.3 ± 0.9 |
5.6 – 8.0 |
7.1 ± 0.8 |
4.0 – 8.0 |
6.2 ± 1.0 |
|
Free CO2 (mg/l) |
6.0 – 24.0 |
13.6 ± 4.0 |
6.0 – 16.0 |
10.2 ± 2.8 |
6.0 – 20.0 |
13.8 ± 3.4 |
|
Alkalinity (mgl-1) |
44.0 – 126.0 |
70.3 ± 20.7 |
64.0 – 116.0 |
92.3 ± 14.2 |
38.0 – 88.0 |
62.2 ± 13.4 |
|
Hardness (mg/l) |
42.0 – 128.0 |
69.8 ± 20.3 |
56.0 – 122.0 |
89.3 ± 16.9 |
38.0 – 84.0 |
60.8 ± 13.6 |
|
Calcium (mg/l) |
27.3 - 81.9 |
43.0 ± 13.1 |
37.8 – 73.5 |
60.2 ± 9.2 |
25.2 – 54.6 |
38.7 ± 7.8 |
|
Magnesium (mg/l) |
1.34 – 11.91 |
6.51 ± 2.81 |
2.19 – 11.88 |
7.08 ± 2.41 |
1.02 – 11.30 |
5.38 ± 2.34 |
|
Chloride (mg/l) |
5.99 – 32.97 |
10.99 ± 5.25 |
3.99 – 21.98 |
8.91 ± 3.49 |
6.99 – 39.96 |
13.15 ± 6.54 |
|
DOM (mg/l) |
0.041 – 0.319 |
0.162 ± 0.062 |
0.026 – 0.278 |
0.113 ± 0.047 |
0.038 – 0.353 |
0.166 ± 0.063 |
|
TDS (mg/l) |
0.088 – 0.172 |
0.137 ± 0.023 |
0.080 – 0.160 |
0.115 ± 0.022 |
0.104 – 0.180 |
0.147 ± 0.020 |
|
Phosphate (mg/l) |
0.145 – 3.619 |
0.963 ± 0.697 |
0.093 – 1.582 |
0.761 ± 0.393 |
0.165 – 1.499 |
0.845 ± 0.414 |
|
Nitrate (mg/l) |
0.501 – 4.522 |
1.855 ± 1.047 |
0.544 – 4.411 |
1.800 ± 1.030 |
0.499 – 3.566 |
1.758 ± 0.838 |
|
Sulphate (mg/l) |
1.387 – 17.776 |
8.789 ± 4.161 |
0.793 – 14.075 |
6.473 ± 3.741 |
0.925 – 13.282 |
7.219 ± 3.600 |
|
Silicate (mg/l) |
0.140 – 2.652 |
0.880 ± 0.547 |
0.140 – 2.547 |
0.825 ± 0.511 |
0.140 – 1.187 |
0.660 ± 0.275 |
Table 1 Abiotic factors of Majuli beels (September 2010-August 2012)
|
Taxa↓ |
Bhereki |
Ghotonga |
Holmari |
|
Phylum: Rotifera |
|
|
|
|
Order: Ploima |
|
|
|
|
Family: Asplanchnidae |
|
|
|
|
1. Asplanchna priodonta Gosse |
+ |
+ |
+ |
|
Family: Brachionidae |
|||
|
2. Anuraeopsis fissa Gosse |
+ |
+ |
- |
|
3. Brachionus angularis Gosse |
- |
- |
- |
|
4. Brachionus durgae Dhanapathi |
- |
- |
- |
|
5. Brachionus falcatus Zacharias |
- |
- |
- |
|
6. Brachionus kostei Shiel |
- |
- |
- |
|
7. Brachionus quadridentatus Hermann |
+ |
+ |
+ |
|
8. Keratella cochlearis (Gosse) |
+ |
+ |
+ |
|
9. Keratella edmondsoni Ahlstrom |
- |
- |
- |
|
10. Keratella lenzi Hauer |
+ |
+ |
- |
|
11. Keratella tecta (Gosse) |
- |
+ |
- |
|
12. Keratella tropica (Apstein) |
+ |
- |
+ |
|
13. Platyias quadricornis (Ehrenberg) |
+ |
+ |
+ |
|
14. Plationus patulus (O.F. Muller) |
+ |
+ |
+ |
|
Family: Euchlanidae |
|||
|
15. Beauchampiella eudactylota (Gosse) |
+ |
+ |
+ |
|
16. Dipleuchlanis propatula (Gosse) |
+ |
- |
+ |
|
17. Euchlanis dilatata Ehrenberg |
+ |
+ |
+ |
|
18. Euchlanis triquetra Ehrenberg |
+ |
+ |
- |
|
19. Tripleuchlanis plicata (Levander) |
- |
+ |
+ |
|
Family: Flosculariidae |
|||
|
20. Sinantherina socialis (Linne) |
- |
+ |
- |
|
21. Sinantherina spinosa (Thorpe) |
+ |
+ |
+ |
|
Family: Lecanidae |
|||
|
22. Lecane aculeata (Jakubski) |
+ |
+ |
+ |
|
23. Lecane arcula Harring |
- |
- |
- |
|
24. Lecane bifurca (Bryce) |
- |
- |
- |
|
25. Lecane blachei Berzins |
- |
+ |
+ |
|
26. Lecane bulla (Gosse) |
+ |
+ |
+ |
|
27. Lecane closterocerca (Schmarda) |
+ |
+ |
+ |
|
28. Lecane crepida Harring |
- |
+ |
+ |
|
29. Lecane curvicornis (Murray) |
+ |
+ |
- |
|
30. Lecane decipiens (Murray) |
- |
- |
- |
|
31. Lecane doryssa Harring |
+ |
+ |
- |
|
32. Lecane flexilis (Gosse) |
- |
- |
- |
|
33. Lecane furcata (Murray) |
+ |
+ |
+ |
|
34. Lecane haliclysta Harring & Myers |
- |
- |
- |
|
35. Lecane hamata (Stokes) |
+ |
+ |
+ |
|
36. Lecane hornemanni (Ehrenberg) |
+ |
+ |
+ |
|
37. Lecane inermis (Bryce) |
+ |
+ |
+ |
|
38. Lecane inopinata Harring & Myers |
+ |
+ |
- |
|
39. Lecane lateralis Sharma |
+ |
+ |
+ |
|
40. Lecane leontina (Turner) |
+ |
+ |
+ |
|
41. Lecane ludwigii (Eckstein) |
+ |
+ |
+ |
|
42. Lecane luna (O.F. Müller) |
+ |
+ |
+ |
|
43. Lecane lunaris (Ehrenberg) |
+ |
+ |
+ |
|
44. Lecane monostyla (Daday) |
- |
+ |
- |
|
45. Lecane nitida (Murray) |
- |
+ |
+ |
|
46. Lecane niwati Segers, Kotethip & Sanoamuang |
- |
- |
- |
|
47. Lecane obtusa (Murray) |
+ |
+ |
+ |
|
48. Lecane ohioensis (Herrick) |
+ |
+ |
- |
|
49. Lecane papuana (Murray) |
+ |
+ |
+ |
|
50. Lecane paxiana Hauer |
- |
- |
- |
|
51. Lecane ploenensis (Voigt) |
+ |
+ |
+ |
|
52. Lecane pusilla Harring |
- |
+ |
+ |
|
53. Lecane pyriformis (Daday) |
+ |
+ |
+ |
|
54. Lecane quadridentata (Ehrenberg) |
+ |
+ |
+ |
|
55. Lecane rhytida Harring & Myers |
- |
- |
- |
|
56. Lecane signifera (Jennings) |
+ |
+ |
+ |
|
57. Lecane stenroosi (Meissner) |
+ |
+ |
+ |
|
58. Lecane styrax (Harring & Myers) |
- |
- |
- |
|
59. Lecane tenuiseta Harring |
- |
- |
- |
|
60. Lecane thienemanni (Hauer) |
- |
- |
- |
|
61. Lecane undulata Hauer |
- |
+ |
- |
|
62. Lecane unguitata (Fadeev) |
+ |
+ |
+ |
|
63. Lecane ungulata (Gosse) |
+ |
+ |
+ |
|
Family: Lepadellidae |
|||
|
64. Colurella adriatica Ehrenberg |
- |
- |
- |
|
65. Colurella obtusa (Gosse) |
+ |
+ |
+ |
|
66. Colurella uncinata (O.F. Müller) |
+ |
+ |
+ |
|
67. Lepadella acuminata(Ehrenberg) |
+ |
+ |
+ |
|
68. Lepadella apsida Harring |
+ |
+ |
- |
|
69. Lepadella benjamini Harring |
+ |
+ |
+ |
|
70. Lepadella biloba Hauer |
- |
- |
- |
|
71. Lepadella costatoides Segers |
- |
- |
- |
|
72. Lepadella dactyliseta (Stenroos) |
- |
- |
- |
|
73. Lepadella discoidea Segers |
+ |
+ |
+ |
|
74. Lepadella elongata Koste |
+ |
+ |
- |
|
75. Lepadella lindaui Koste |
- |
- |
- |
|
76. Lepadella minuta (Weber & Montet) |
- |
- |
- |
|
77. Lepadella ovalis (O.F. Muller) |
+ |
+ |
+ |
|
78. Lepadella patella (O.F. Muller) |
+ |
+ |
+ |
|
79. Lepadella quinquecostata (Lucks) |
- |
- |
- |
|
80. Lepadella rhomboides (Gosse) |
+ |
+ |
+ |
|
81. Lepadella triba Myers |
- |
- |
- |
|
82. Lepadella triptera Ehrenberg |
- |
- |
- |
|
83. Lepadella vandenbrandei Gillard |
- |
- |
- |
|
84. Lepadella (Heterolepadella) apsicora Myers |
- |
- |
- |
|
85. Lepadella (H.) ehrenbergi Perty |
+ |
+ |
+ |
|
86. Lepadella (H.) heterostyla (Murray) |
- |
+ |
- |
|
Family: Mytilinidae |
|||
|
87. Lophocharis oxysternon (Gosse) |
- |
- |
+ |
|
88. Mytilina acanthophora Hauer |
+ |
+ |
- |
|
89. Mytilina bisulcata (Lucks) |
+ |
- |
+ |
|
90. Mytilina michelangellii Reid & Turner |
- |
- |
- |
|
91. Mytilina ventralis (Ehrenberg) |
+ |
+ |
+ |
|
Family: Notommatidae |
|||
|
92. Cephalodella forficula (Ehrenberg) |
- |
+ |
+ |
|
93. Cephalodella gibba ( Ehrenberg) |
+ |
+ |
+ |
|
94. Monommata longiseta (O.F. Müller) |
+ |
+ |
+ |
|
Family: Scaridiidae |
|||
|
95. Scaridium longicaudum ( Müller) |
+ |
+ |
+ |
|
Family: Synchaetidae |
|||
|
96. Pleosoma lenticulare Herrick |
- |
- |
- |
|
97. Polyarthra vulgaris Carlin |
+ |
+ |
+ |
|
Order: Flosculariaceae |
|||
|
Family: Conochilidae |
|||
|
98. Conochilus unicornis Rousselet |
- |
- |
- |
|
Family: Hexarthridae |
|||
|
99. Hexarthra mira (Hudson) |
- |
- |
- |
|
Family: Testudinellidae |
|||
|
100. Testudinella amphora Hauer |
- |
- |
- |
|
101. Testudinella emarginula Stenroos |
+ |
+ |
+ |
|
102. Testudinella patina (Hermann) |
+ |
+ |
+ |
|
103. Testudinella tridentata Smirnov |
- |
- |
- |
|
104. Pompholyx sulcata Hudson |
+ |
+ |
+ |
|
Family: Trichocercidae |
|||
|
105. Trichocerca bicristata (Gosse) |
- |
- |
- |
|
106. Trichocerca cylindrica (Imhof) |
+ |
+ |
+ |
|
107. Trichocerca elongata (Gosse) |
- |
- |
- |
|
108. Trichocerca insignis (Herrick) |
+ |
+ |
+ |
|
109. Trichocerca rattus (O.F. Muller) |
+ |
+ |
+ |
|
110. Trichocerca scipio (Gosse) |
- |
- |
- |
|
111. Trichocerca similis (Wierzejski) |
+ |
+ |
+ |
|
112. Trichocerca tigris (O.F. Muller) |
+ |
+ |
- |
|
113. Trichocerca uncinata (Voigt) |
- |
- |
- |
|
114. Trichocerca weberi (Jennings) |
- |
- |
- |
|
Family: Trichotriidae |
|||
|
115. Macrochaetus longipes Myers |
- |
+ |
+ |
|
116. Macrochaetus sericus (Thorpe) |
+ |
+ |
+ |
|
117. Trichotria tetractis (Ehrenberg) |
+ |
+ |
+ |
|
Family: Trochosphaeridae |
|||
|
118. Filinia camasecla Myers |
- |
- |
- |
|
119. Filinia longiseta (Ehrenberg) |
+ |
+ |
+ |
|
120. Trochosphaera aequatorialis Semper |
- |
- |
- |
|
Sub-class: Digononta |
|||
|
Order: Bdelloidea |
|||
|
Family: Philodinidae |
|||
|
121. Philodina citrina Ehrenberg |
+ |
+ |
+ |
|
122. Rotaria neptunia (Ehrenberg) |
+ |
+ |
- |
|
123. Rotaria rotatoria (Pallas) |
- |
- |
+ |
|
Super-order: Cladocera (sensu strictu) |
|||
|
Family: Bosminidae |
|||
|
124. Bosmina longirostris Sars s.lat |
- |
+ |
- |
|
125. Bosminopsis deitersi Richard |
- |
- |
- |
|
Family: Chydoridae |
|||
|
Subfamily: Aloninae |
|||
|
126. Alona affinis (Leydig) s.lat |
- |
- |
- |
|
127. Alona cheni Sinev |
- |
+ |
- |
|
128. Alona guttata tuberculata Kurz |
+ |
- |
+ |
|
129. Alona kotovi Sinev |
- |
- |
- |
|
130 Anthalona harti Van Damme et al. |
- |
- |
- |
|
131. Camptocercus uncinatus Smirnov |
+ |
+ |
- |
|
132. Celsinotum macronyx (Daday) |
- |
+ |
- |
|
133. Celsinotum rectangula (Sars) s.lat |
+ |
- |
+ |
|
134. Euryalona orientalis (Daday) |
- |
+ |
+ |
|
135. Graptoleberis testudinaria (Fischer) |
- |
+ |
+ |
|
136. Karualona karua (King) |
+ |
+ |
+ |
|
137. Kurzia brevilabris Rajapaksa & Fernando |
- |
- |
- |
|
138. Kurzia latissima Kurz |
- |
+ |
+ |
|
139. Kurzia longirostris (Daday) |
+ |
+ |
- |
|
140. Leberis diphanus (King) |
- |
- |
- |
|
141. Notoalona globulosa (Daday) |
+ |
+ |
+ |
|
142. Oxyurella singalensis (Daday) |
- |
- |
- |
|
Subfamily: Chydorinae |
|||
|
143. Alonella clathratula Sars |
- |
+ |
+ |
|
144. Alonella excisa (Fischer) |
+ |
+ |
+ |
|
145. Chydorus angustirostris Frey |
+ |
+ |
- |
|
146. Chydorus sphaericus (O. F. Muller) |
+ |
+ |
+ |
|
147. Chydorus ventricosus Daday |
- |
+ |
+ |
|
148. Dadaya macrops (Daday) |
- |
- |
- |
|
149. Disperalona caudata Smirnov |
+ |
- |
+ |
|
150. Dunhevedia crassa King |
- |
- |
- |
|
151. Dunhevedia serrata Daday |
+ |
+ |
- |
|
152. Picripleuroxus quasidenticulatus (Smirnov) |
- |
- |
- |
|
153. Picripleuroxus similis Vavra |
+ |
+ |
+ |
|
Family: Daphniidae |
|||
|
154. Ceriodaphnia cornuta Sars |
+ |
+ |
+ |
|
155. Scapholeberis kingi Sars |
+ |
+ |
- |
|
156. Simocephalus acutirostratus(King) |
- |
- |
+ |
|
157. Simocephalus serrulatus (Koch) |
+ |
+ |
+ |
|
158. Simocephalus mixtus Sars |
+ |
+ |
- |
|
Family: Ilyocryptidae |
|||
|
159. Ilyocryptus spinifer Herrick |
- |
- |
- |
|
Family: Macrothricidae |
|||
|
160. Grimaldina brazzai Richard |
- |
- |
- |
|
161. Guernella raphaelis Richard |
- |
- |
+ |
|
162. Macrothrix laticornis (Fischer) |
+ |
+ |
+ |
|
163. Macrothrix spinosa King |
- |
- |
- |
|
164. Macrothrix triserialis (Brady) |
+ |
+ |
+ |
|
Family: Moinidae |
|||
|
165. Moina micrura Kurz |
- |
+ |
+ |
|
166. Moinodaphnia macleayi (King) |
- |
+ |
+ |
|
Order: Ctenopoda |
|||
|
Family: Sididae |
|||
|
167. Diaphanosoma excisum Sars |
+ |
+ |
+ |
|
168. Diaphanosoma sarsi Richard |
+ |
- |
- |
|
169. Diaphanosoma senegal Gauthier |
- |
- |
- |
|
170. Pseudosida szalayi (Daday) |
- |
- |
- |
|
171. Sida crystallina (O. F. Muller) |
- |
+ |
+ |
|
Sub-Kingdom: Protozoa |
|||
|
Super-class: Rhizopoda |
|||
|
172. Arcella discoides Ehrenberg |
+ |
+ |
+ |
|
173. Arcella hemispherica Perty |
+ |
+ |
+ |
|
174. Arcella vulgaris Ehrenberg |
+ |
+ |
+ |
|
Family: Centropyxidae |
|||
|
175. Centropyxis aculeata (Ehrenberg) |
+ |
+ |
+ |
|
176. Centropyxis ecornis (Ehrenberg) |
+ |
+ |
+ |
|
177. Centropyxis oblonga (Deflandre) |
+ |
+ |
+ |
|
Family: Difflugidae |
|||
|
178. Difflugia acuminata Ehrenberg |
+ |
+ |
+ |
|
179. Difflugia corona Wallich |
+ |
+ |
+ |
|
180. Difflugia oblonga Ehrenberg |
+ |
+ |
+ |
|
181. Difflugia urceolata Carter |
- |
+ |
+ |
|
Family: Euglyphidae |
|||
|
182. Assulina muscorum Greet |
+ |
+ |
+ |
|
183. Euglypha acanthophora Dujardin |
+ |
+ |
+ |
|
184. E. tuberculata Dujardin |
- |
+ |
+ |
|
185. Trinema enchelys (Ehrenberg) |
- |
- |
+ |
|
Family: Nebelidae |
|||
|
186. Lesquereusia spiralis (Ehrenberg) |
+ |
+ |
+ |
|
187. Nebela caudata Leidy |
+ |
+ |
+ |
|
Class: Copepoda |
|||
|
Family: Cyclopidae |
|||
|
188. Tropocyclops prasinus (Fischer) |
+ |
+ |
+ |
|
189. Mesocyclops leuckarti (Claus) |
+ |
+ |
+ |
|
190. Microcyclops varicans Sars |
+ |
+ |
+ |
|
191. Thermocyclops decipiens |
+ |
+ |
+ |
|
Family: Diaptomidae |
|||
|
192. Heliodiaptomus cinctus |
+ |
+ |
+ |
|
193. Neodiaptomus schmackeri |
+ |
+ |
+ |
|
Class: Ostracoda |
|||
|
Family: Cyprididae |
|||
|
194. Cypris subglobulosa |
+ |
+ |
+ |
|
195. Strandesia indica |
+ |
+ |
+ |
|
196. Hemicypris anomala |
- |
- |
- |
|
Species Richness |
|||
|
Rotifera |
70 |
79 |
66 |
|
Cladocera |
20 |
27 |
23 |
|
Rhizopoda |
13 |
15 |
16 |
|
Copepoda |
6 |
6 |
6 |
|
Ostracoda |
2 |
2 |
2 |
|
Total Zooplankton |
111 |
129 |
113 |
Table 2 Species composition of zooplankton of Majuli beels
|
Taxa↓ beels → |
Bhereki beel |
Holmari beel |
Ghotonga beel |
|||
|
Qualitative |
||||||
|
Net Plankton richness |
209 species |
212 species |
232 species |
|||
|
Zooplankton richness |
111 species |
113 species |
129 species |
|||
|
Percentage similarity % |
48.8 – 75.7 |
49.1 – 74.3 |
46.1 – 69.7 |
|||
|
Zooplankton (species) |
45 – 64 |
54 ± 6 |
47 – 67 |
57 ± 6 |
49 – 76 |
63 ± 8 |
|
Rotifera |
21 – 35 |
28 ± 4 |
22 – 39 |
28 ± 4 |
23 – 45 |
33 ± 6 |
|
Cladocera |
5 – 14 |
10 ± 2 |
4 – 14 |
11 ± 3 |
8 – 18 |
13 ± 3 |
|
Quantitative |
||||||
|
Net Plankton (nl-1) |
261 – 1253 |
663 ± 261 |
449 – 1815 |
682 ± 289 |
282 – 1923 |
628 ± 320 |
|
Zooplankton (nl-1) |
173 – 388 |
245 ± 52 |
163 – 523 |
275 ± 87 |
187 – 448 |
293 ± 71 |
|
% composition |
23.8 – 73.2 |
40.5 ± 12.5 |
15.2 – 61.0 |
42.9 ± 12.3 |
15.4 – 78.0 |
51.4 ± 13.5 |
|
Diversity |
3.012 – 3.793 |
3.555 ± 0.184 |
3.245 – 4.042 |
3.650 ± 0.197 |
3.464 – 4.111 |
3.813 ± 0.172 |
|
Dominance |
0.063 – 0.196 |
0.115 ± 0.036 |
0.048 – 0.174 |
0.099 ± 0.031 |
0.042 – 0.139 |
0.082 ± 0.028 |
|
Evenness |
0.755 – 0.944 |
0.893 ± 0.041 |
0.803 – 0.962 |
0.905 ± 0.039 |
0.877 – 0.970 |
0.924 ± 0.032 |
|
Rotifera (nl-1) |
44 – 132 |
80 ± 22 |
48 – 179 |
89 ± 32 |
61 – 221 |
119 ± 37 |
|
% composition |
23.9 – 52.0 |
32.8 ± 6.5 |
11.9 – 51.7 |
34.0 ± 10.1 |
25.8 – 52.4 |
40.4 ± 6.7 |
|
Cladocera (nl-1) |
15 – 99 |
38 ± 20 |
12 – 59 |
33 ± 14 |
24 – 116 |
52 ± 21 |
|
% composition |
7.7 – 28.2 |
15.3 ± 6.2 |
6.5 – 18.9 |
11.9 ± 3.5 |
10.4 – 28.8 |
17.7 ± 5.1 |
|
Rhizopoda (nl-1) |
22 – 133 |
72 ± 31 |
39 – 254 |
99 ± 57 |
39 – 141 |
76 ± 26 |
|
% composition |
8.4 – 47.0 |
29.5 ± 11.0 |
16.1 – 65.2 |
34.3 ± 11.6 |
12.5 – 37.8 |
26.4 ± 7.5 |
|
Copepoda (nl-1) |
13 – 154 |
52 ± 32 |
12 – 120 |
50 ± 26 |
17 – 108 |
42 ± 23 |
|
% composition |
4.6 – 56.4 |
21.2 ± 11.9 |
3.8 – 43.8 |
18.4 ± 8.7 |
7.5 – 32.6 |
14.1 ± 6.1 |
|
Ostracoda (nl-1) |
0 – 9 |
3 ± 2 |
1 – 10 |
4 ± 3 |
0 – 8 |
4 ± 3 |
|
Important families (nl-1) |
||||||
|
Lecanidae |
24 – 60 |
37 ± 9 |
18 – 106 |
40 ± 21 |
12 – 80 |
44 ± 15 |
|
Lepadellidae |
4 – 23 |
12 ± 5 |
6 – 21 |
11 ± 4 |
6 – 30 |
17 ± 7 |
|
Brachionidae |
3 – 32 |
10 ± 6 |
2 – 19 |
8 ± 5 |
2 – 64 |
22 ± 20 |
|
Chydoridae |
8 – 65 |
20 ± 11 |
7 – 35 |
19 ± 8 |
15 – 84 |
30 ± 16 |
|
Arcellidae |
6 – 77 |
39 ± 22 |
8 – 104 |
28 ± 21 |
13 – 53 |
30 ± 11 |
|
Centropyxidae |
5 – 29 |
11 ± 6 |
3 – 52 |
23 ± 15 |
1 – 62 |
16 ± 4 |
|
Difflugidae |
0 – 23 |
10 ± 7 |
3 – 42 |
14 ± 9 |
1 – 32 |
12 ± 8 |
|
Euglephidae |
0 – 14 |
5 ± 4 |
1 – 69 |
18 ± 16 |
1 – 25 |
9 ± 7 |
|
Macrothricidae |
0 – 28 |
7 ± 7 |
0 – 17 |
5 ± 4 |
0 – 28 |
11 ± 8 |
|
Important species (nl-1) |
||||||
|
Arcella discoides |
0 – 36 |
16 ± 10 |
2 – 40 |
11 ± 9 |
5 – 28 |
13 ± 6 |
|
Tropocyclops prasinus |
4 – 53 |
20 ± 14 |
2 – 40 |
16 ± 10 |
3 – 41 |
14 ± 10 |
|
A. vulgaris |
0 – 40 |
14 ± 10 |
2 – 63 |
12 ± 13 |
4 – 34 |
10 ± 6 |
|
Centropyxis aculeata |
1 – 15 |
5 ± 4 |
0 – 30 |
14 ± 9 |
0 – 40 |
10 ± 9 |
|
Euglypha acanthophora |
0 – 14 |
4 ± 4 |
0 – 55 |
10 ± 12 |
0 – 20 |
6 ± 5 |
|
Nebela caudata |
1 – 16 |
7 ± 4 |
2 – 42 |
11 ± 10 |
3 – 16 |
8 ± 3 |
|
Thermocyclops decipiens |
5 – 51 |
17 ± 11 |
0 – 30 |
6 ± 7 |
0 – 29 |
8 ± 7 |
|
Mesocyclops leuckarti |
0 – 32 |
9 ± 7 |
2 – 34 |
16 ± 9 |
3 – 30 |
10 ± 8 |
|
Microcyclops varicans |
4 – 18 |
5 ± 4 |
1 – 34 |
10 ± 7 |
0 – 29 |
8 ± 7 |
|
Macrothrix triserialis |
0 – 22 |
6 ± 6 |
0 – 4 |
1 ± 1 |
0 – 28 |
10 ± 8 |
Table 3 Temporal variations of zooplankton (September 2010-August, 2012)
Zooplankton density (Table 3) ranged between 173–388, 163–523 and 187–448 n/l (Figures 10 & 11); it comprised 40.5±12.5, 42.9±12.3 and 51.4±13.5 % of net plankton of Bhereki, Holmari and Ghotonga beels, respectively. Rotifera density varied between 44–132, (80±22), 48–179 (89±32) and 61–221 (119 ±37) n/l and comprised 32.8±6.5, 34.0±10.1 and 40.4±6.7% (Table 2) of zooplankton; Rhizopoda showed density variations between 22–133 (72±31), 39–254 (99±57) and Ghotonga 39–141 (76±26) n/l and comprised 29.5±11.0, 34.3±11.6 and 26.4±7.5 % of zooplankton; Copepoda density varied between 13–154 (52±32), 12–120 (50±26) and 17–108 (42±23) n/l and comprised between 21.2±11.9, 18.4±8.7 and 7.5–14.1±6.1 % of zooplankton; and Cladocera abundance varied between 15–99 (38±20), 12–59 (33±14) and 24–116 ( 52±21) n/l and formed between 15.3±6.2, 11.9±3.5 and 17.7±5.1 % of zooplankton abundance of three beels, respectively (Table 3). The species diversity, dominance and evenness varied (Table 2) between 3.012–3.793, 3.245–4.042 and 3.464–4.111 (Figures 12 & 13); 0.063–0.196, 0.048–0.174 and 0.042–0.139; 0.755–0.944, 0.803–0.962 and 0.877–0.970 in the sampled beels, respectively. The CCA ordination biplots of zooplankton assemblages and abiotic factors of three beels are indicated in Figures14-16, respectively.
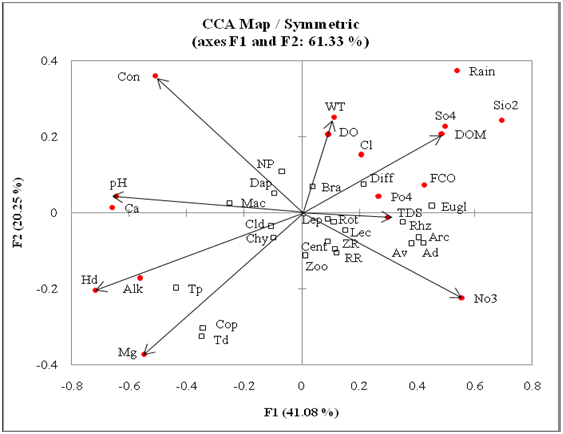
Figure 13 CCA ordination biplot of Zooplankton assemblages and environmental variables (Bhereki beel).
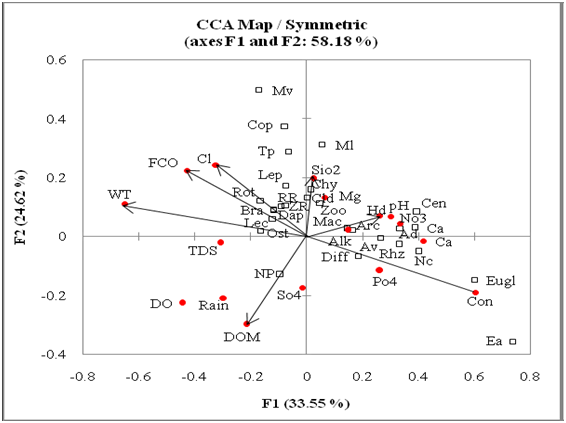
Figure 14 CCA ordination biplot of Zooplankton assemblages and environmental variables (Holmari beel).

Figure 15 CCA ordination biplot of Zooplankton assemblages and environmental variables (Ghotonga beel).
Abiotic: ALK: Alkalinity; Ca: Calcium; Cl: Chloride; CON: Conductivity; DO: Dissolved Oxygen; DOM: Dissolved Oxygen Matter; FCO: Free Carbon Dioxide; HD: Hardness; Mg: Magnesium; pH Hydrogen-Ion Concentration; NO3: Nitrate; PO4: Phosphate; Rain: Rainfall; SIO2: Silicate; SO4: Sulphate; TDS: Total Dissolved Solids; WT: Water Temperature
Biotic: AD: Arcella discoides; ARC: Arcellidae; Av: A. vulgaris; BRA: Brachionidae; CEN: Centropyxidae; CHY: Chydoridae; CLD: Cladocera; COP: Copepoda; DAP: Daphniidae; DIFF: Difflugidae; EUGL: Euglephidae; LEC: Lecanidae; LEP: Lepadellidae; MAC: Macrothricidae; NP: Net Plankton; ORHZ: Rhizopoda; ROT: Rotifera; RR: Rotifera Richness; TD: Thermocyclops Decipiens; TP: Tropocyclops Prasinus; ZP: Zooplankton; ZR: Zooplankton Richness
Abbreviations: Abiotic: ALK: Alkalinity; Ca: Calcium; Cl: Chloride; CON: Conductivity; DO: Dissolved oxygen; DOM: Dissolved Oxygen Matter; FCO: Free Carbon Dioxide; HD: Hardness; Mg: Magnesium; pH Hydrogen-ion concentration; NO3: Nitrate; PO4: phosphate; Rain: rainfall; SIO2: silicate; SO4: sulphate; TDS: Total dissolved solids; WT: water temperature
Biotic: AD: Arcella discoides; ARC: Arcellidae; Av: A. vulgaris; BRA: Brachionidae; CEN: Centropyxidae; CHY: Chydoridae; CLD: Cladocera; COP: Copepoda; DAP: Daphniidae; DIFF: Difflugidae; EUGL: Euglephidae; LEC: Lecanidae; LEP: Lepadellidae; MAC: Macrothricidae; NP: Net plankton; ORHZ: Rhizopoda; ROT: Rotifera; RR: Rotifera richness; TD: Thermocyclops decipiens; TP: Tropocyclops prasinus; ZP: Zooplankton; ZR: Zooplankton richness
Abbreviations: Abiotic: ALK: Alkalinity; Ca: Calcium; Cl: Chloride; CON: Conductivity; DO: Dissolved oxygen; DOM: Dissolved Oxygen Matter; FCO: Free Carbon Dioxide; HD: Hardness; Mg: Magnesium; pH Hydrogen-ion concentration; NO3: Nitrate; PO4: phosphate; Rain: rainfall; SIO2: silicate; SO4: sulphate; TDS: Total dissolved solids; WT: water temperature
Biotic: AD: Arcella discoides; ARC: Arcellidae; Av: A. vulgaris; BRA: Brachionidae; CEN: Centropyxidae; CHY: Chydoridae; CLD: Cladocera; COP: Copepoda; DAP: Daphniidae; DIFF: Difflugidae; EUGL: Euglephidae; LEC: Lecanidae; LEP: Lepadellidae; MAC: Macrothricidae; Ml: Mesocyclops leuckarti; MT: Macrothrix triserialis; NP: Net plankton; RHZ: Rhizopoda; ROT: Rotifera; RR: Rotifera richness; TP: Tropocyclops prasinus; ZP: Zooplankton; ZR: Zooplankton richness
Abiotic parameters
Water temperature concurred with the geographical location of the sampled beels. Bhereki and Holmari beels are characterized by slightly acidic to circum-neutral waters whereas Ghotonga beel indicated slightly acidic waters. The specific conductivity exhibited low ionic concentration of the three beels and warranted of these wetlands under ‘Class I’ category of trophic classification.22 All three floodplain lakes are characterized by moderately hard water character, moderate dissolved oxygen, low free CO2, low chloride content, and relatively low concentrations of dissolved organic matter and total dissolved solids.
Zooplankton richness
Zooplankton (141 species) of Majuli beels are more biodiverse than the reports from various beels of Assam,1 two floodplain lakes of Manipur,8 certain lakes of Kashmir Himalayas,23,24 two Kumaun lakes of Uttarakhand,25,26 two wetlands of Barak river basin of Assam,27 two floodplain lakes of southwest Bengal28 and from two wetlands of Kashmir.29 The zooplankton richness of individual Majuli beels (118±8 species) is yet relatively lower than the report of 143 species enlisted from Ghorajan beel of Assam7 it is reasonably comparable with 123 species known from a sub-tropical lake of Jammu province30 while it is higher than 93 species known from Dal Lake31 and 85 species from Wular wetland32 of Kashmir; and 70 species from a floodplain wetland of West Bengal.33
Zooplankton contributed significantly to net plankton richness in Bhereki (r1= 0.732, p < 0.0001), Holmari (r2= 0.705, p = 0.0001) and Ghotonga (r3= 0.749, p < 0.0001) beels. The monthly richness followed broadly concurrent variations in Bhereki (54±6 species) and Holmari (57±6 species) beels than marginally high richness in Ghotonga beel (63±8 species). ANOVA indicated significant richness variations amongst three beels (F2,71 = 11.201, P = 0.0001). The community similarities (vide Sørensen’s index) of 48.8–75.7%, 49.1–77.6% and 46.1–69.7% in Bhereki, Holmari and Ghotonga beels, respectively coupled with monthly richness variations suggested heterogeneity in zooplankton composition in individual beels. The hierarchical cluster analysis of Bhereki beel indicated high zooplankton affinities between June vs. July during the first year; and between July vs. August during second year while peak divergence is noticed during February and September > February > October during two years, respectively. In Holmari beel, maximum affinity was recorded between January vs. February and April vs. May collections while November > September > May and February > January > June communities indicated high divergence during two years of the study period, respectively. Further in Ghotonga beel, high affinities are indicated between January vs. May and again between October vs. July communities during first year and between July vs. August communities during second year while maximum divergence is noted during April and September collections during two years, respectively. The cluster groupings indicated distinct annual variations, during two years, in three beels individually and thus affirmed heterogeneity in monthly composition of zooplankton communities.
The richness followed oscillating temporal variations in the sampled beels concurrent with the reports of Sharma and Sharma7 and Sharma6,8 while it differed from winter and autumn maxima reported from Loktak Lake, Manipur.9 Peak richness of 64, 67 and 76 species was observed during winter (February, 2012), monsoon (August, 2012) and winter (January, 2012) in Bhereki, Holmari and Ghotonga beels, respectively. Rotifera (84 species), the most species-rich group of Ghotonga > Bhereki > Holmari beels contributed significantly to temporal variations of zooplankton richness (r1 = 0.884, p < 0.0001; r2 = 0.661, p = 0.0003; r3 = 0.875, p < 0.0001). Besides, Cladocera contributed significantly to zooplankton richness in the sampled beels (r1 = 0.736, p < 0.0001; r2 = 0.782, p < 0.0001; r3 = 0.804, p < 0.0001). More remarks on the diversity of the stated groups are made separately.11,12
Zooplankton abundance was relatively higher in Ghotonga beel (293±71 n/l) than that of Bhereki (245±52 n/l) and Holmari (275±87 n/l) beels; it registered insignificant annual as well as monthly density variations amongst the three beels as well as in the individual beels. The recorded zooplankton abundance concurred with the reports from Ghorajan beel of Assam,7 and two floodplain lakes of Manipur.8,9 Further, the recorded densities are lower than the reports from Surinsar lake of Kashmir,30 Deepor Beel – a Ramsar site6 of Assam, and the results from various Indian floodplain lakes.28, 29, 34-36 Zooplankton followed oscillating patterns of monthly density variations with peaks during post-monsoon (October, 2010), summer (April, 2012) and winter (January, 2012) in Bhereki, Holmari and Ghotonga beels, respectively. The winter peak of Ghotonga beel concurred with reports of Sharma6-8 and Sharma and Sharma9 while oscillating patterns agreed with the report of Sharma and Sharma12 but differed from bimodal pattern noted by Sanjer and Sharma35 but. Zooplankton formed the dominant quantitative component of net plankton in Ghotonga beel (51.4±13.5%) while it formed the sub-dominant component (40.5±12.5% and 42.9±12.3%) in Bhereki and Holmari beels respectively. The dominance in Ghotonga beel suggested availability of other food resources such as organic matter absorbed in sediments, detritus and bacteria.7
Interestingly, this study indicated differences in quantitative importance of zooplankton groups in different beels during the study and also during two successive years. Rotifera > Rhizopoda > Copepoda > Cladocera in Bhereki beel; Rhizopoda > Rotifera > Copepoda > Cladocera in Holmari beel; and Rotifera > Rhizopoda > Cladocera > Copepoda in Ghotonga beel, in the stated order, contributed to zooplankton during the study period. On the other hand, Rotifera > Copepoda > Rhizopoda > Cladocera and Rhizopoda > Rotifera > Copepoda > Cladocera indicated importance in Bhereki beel during two years, respectively. In Holmari beel, Rotifera > Rhizopoda > Copepoda > Cladocera contributed to zooplankton during first year while Rhizopoda > Rotifera > Copepoda > Cladocera showed importance during second year. Further, Rotifera > Rhizopoda > Copepoda ≥ Cladocera contributed to zooplankton abundance during first year in Ghotonga beel while Rotifera > Rhizopoda ≥ Cladocera > Copepoda deserved mention during second year. The variations are hypothesized to habitat diversity and environmental heterogeneity amongst three beels during the study as well as during two years.
Rotifera, an important group, is characterized by marginal density variations in Bhereki (80±22 n/l), Holmari (89±32 n/l) and Ghotonga (119±37 n/l) beels with higher mean density during first year in Holmari and during second year in other two beels. It formed the dominant component of zooplankton in Bhereki (32.8±6.5%) and Ghotonga (40.4±6.7%) beels while it comprised a sub-dominant group in Holmari beel (34.0±10.1%). The rotifers contributed significantly to zooplankton density variations of Bhereki and Ghotonga (r1 = 0.697, p = 0.0001; r3 = 0.851, p < 0.0001) beels; this generalization is evident from the fact that peak density of Rotifera concurred with zooplankton peak in Ghotonga beel while no such trend was observed in Bhereki beel. ANOVA registered significant rotifer density variations (F2,71 = 10.595, P = 0.0001) amongst three beels. The importance of Rotifera in Bhereki and Ghotonga beels agreed with the reports of1,3,6-9,29,35 while its sub-dominance in Holmari beel agreed with the reports of.5,28,37,38 The Rotifera density followed indefinite monthly variations in the sampled beels with peaks during post-monsoon (September, 2010), summer (July, 2011) and winter (January, 2012) in Bhereki, Holmari and Ghotonga beels, respectively. Their post-monsoon peak concurred with the reports from the floodplains of the Kashmir valley,29 winter peak concurred with the results from certain floodplain lakes of northeast India5, 6, 7, 8 while summer peak concurred with the reports of.35,37 Lecanidae > Lepadellidae contributed notably to Rotifera abundance in Bhereki and Holmari beels while Lecanidae > Brachionidae contributed in Ghotonga beel. The importance of the littoral periphytonic taxa of three Eurotatorien families is attributed to lack of true limnetic conditions in the sampled beels. The lack of dominance of individual rotifer species in any of the sampled lakes suggested that the rotifers are generalists in terms of general environment.3
Rhizopoda, a dominant group of zooplankton of Holmari beel (34.3±11.6%) and a sub-dominant component (29.5±11.0%, 26.4±7.5%) in Bhereki and Ghotonga beels, registered insignificant variations amongst the sampled beels. The rhizopods contributed significantly to zooplankton density only in Holmari beel (r2 = 0.846, p < 0.0001); this generalization is supported by the fact that their maxima contributed to zooplankton peak in this wetland. This group followed no definite pattern of quantitative variations during the study period and recorded peaks during monsoon (August, 2012), pre-monsoon (April, 2012) and autumn (September, 2010) in Bhereki, Holmari and Ghotonga beels, respectively. The present results differed from summer periodicity of these testaceans vide.5,39 Arcellidae > Centropyxidae > Difflugidae contributed to the Rhizopoda abundance in Bhereki and Ghotonga beels, respectively while Arcellidae > Centropyxidae > Euglephidae contributed to their density in Holmari beel. Arcella discoides and A. vulgaris collectively influenced the rhizopod abundance in Bhereki beel; Arcella discoides, A. vulgaris, Centropyxis aculeata, Euglypha acanthophora and Nebela caudata showed importance in Holmari beel; while Arcella discoides, A. vulgaris and Centropyxis aculeata deserved mention in Ghotonga beel.
Copepoda is a sub-dominant group of Bhereki > Holmari > Ghotonga beels; the stated role was in contrast to their dominance reported by.5,28,37 It indicated significant annual (F1,23 = 21.832, P = 0.0006) as well as significant monthly (F11,23 = 4.073, P = 0.014) variations in Bhereki beel. This group registered no definite pattern of monthly density variations during the study in the sampled beels and registered peak values during autumn in Bhereki (November, 2010) and Holmari (October, 2010) beels and during early summer (April, 2011) in Ghotonga beel. Cyclopoids mainly influenced quantitative variations of this group in Bhereki, Holmari and Ghotonga beels, respectively; this reflected the prevalence of stable environmental conditions for these ‘k-strategists’.40,41 Tropocyclops prasinus showed importance in the three beels, respectively; Mesocyclops leuckarti showed certain importance in Holmari > Ghotonga beels while Thermocyclops decipiens deserved mention in Bhereki beel and Microcyclops varicans indicated limited role in Holmari beel. The occurrence of nauplii throughout the study showed an active continuous reproductive phase of the cyclopoids.6,8,9,42
Cladocera formed another sub-dominant group in Ghotonga > Bhereki > Holmari beels respectively, and registered significant density variations amongst three beels (F2,71 = 5.872, P = 0.005). The Cladoceran abundance followed no definite pattern of monthly density variations in the sampled beels and registered peak values during autumn (October, 2010) in Bhereki beel and during winter (January, 2011 and January, 2012) in Holmari and Ghotonga beels, respectively. The winter peaks concurred with the reports of Sharma.6,9 The Cladocera were characterized by importance of Chydoridae in all three beels concurrent with the results of;1,6-9 Daphniidae and Macrothricidae were other important families in the sampled beels while Macrothrix triserialis showed certain value in Ghotonga beel. Ostracoda, another group of zooplankton, indicated very poor abundance in the sampled beels.
Zooplankton of the Majuli beels are characterized by consistently high species diversity throughout the study with higher diversity (> 4.0) during May, 2011 (summer), November, 2011 (autumn) and August, 2012 (monsoon) in Ghotonga beel and during August, 2012 (monsoon) in Holmari beel. The interesting feature is hypothesized to habitat diversity and environmental heterogeneity of the sampled beels. High diversity with lower densities of majority of species in different beels is attributed to fine niche portioning amongst zooplankton species in combination with micro- and macro-scale habitat heterogeneity as hypothesized by Segers H43 and affirmed by.6-9 This generalization is endorsed by relative quantitative importance of only ten out of a total of 141 zooplankton species known from the sampled beels with only three namely Arcella discoides, A vulgaris and Tropocyclops prasinus common to all three beels but in relatively low average densities. The low densities of the rest of species, suggested that the majority of zooplankton is generalists in terms of general environment as hypothesized by Sharma BK.3 ANOVA showed significant diversity variations amongst three beels (F2,23 = 13.046, P = 3.25E-05. The present study did not follow any definite annual and monthly patterns of zooplankton diversity in the three sampled beels.
Lower zooplankton dominance of Bhereki, Holmari and Ghotonga beels is attributed to lack of distinct quantitative importance of different species coupled with low densities of majority of species. The former is hypothesized44 to the fact that the habitat of the sampled Majuli beels had resources for utilization by majority of species and thus providing high amount of niche overlap. This generalization holds valid throughout the study except of limited role of Arcella discoides, A. vulgaris, Tropocyclops prasinus in fewer collections in the three beels; it is affirmed by inverse correlations between dominance vs. diversity in Bhereki (r1 = -0.700 p = 0.0001), Holmari (r2 = -0.880, p < 0.0001) and Ghotonga (r3 = -0.799, p < 0.0001) beels, respectively. High evenness affirmed low densities and equitable abundance of various species and reiterated that the majority of zooplankton are ‘generalists’ vis-à-vis their general environment.3 ANOVA registered significant dominance (F2,71 = 8.009, P = 0.001) and evenness variations (F2,71 = 5.070, P = 0.010) amongst three beels.
This study indicated insignificant influence of individual abiotic parameters on zooplankton richness. Of the different groups, only Rotifera richness is positively correlated with dissolved organic matter (r2 = 0.551, p = 0.0026) in Holmari beel. Zooplankton abundance is inversely correlated with specific conductivity (r3 = -0.598, p = 0.002) in Ghotonga beel; Rhizopoda abundance is inversely correlated with water temperature (r2= -0.556, p = 0.0024) in Holmari beel and directly correlated with pH (r3 = 0.567, p = 0.0019) in Ghotonga beel; Cladocera positively correlated with sulphate (r3 = 0.565, p = 0.002) in Ghotonga beel while Copepoda positively correlated with total hardness (r1 = 0.565, p = 0.002) and magnesium (r1 = 0.555, p = 0.0024) in Bhereki beel and is negatively correlated with total dissolved solids (r3 = -0.540, p = 0.0032) in Ghotonga beel. Rotifera abundance exhibited no significant correlation of any abiotic parameter in the sampled beels. The results thus concluded limited influence of abiotic factors on richness and abundance of zooplankton in this study.
Canonical correspondence analysis (CCA) with 17 abiotic factors recorded lower cumulative influence on zooplankton assemblages along first two axes of 61.33%, 58.18% and 63.77% in Bhereki, Holmari and Ghotonga beels, respectively. The results showed the importance of water temperature, pH, specific conductivity, hardness, magnesium, dissolved organic matter, total dissolved solids and nitrate for zooplankton taxa in Bhereki beel. Water temperature, free carbon-dioxide, specific conductivity, dissolved organic matter, hardness, chloride and silicate reflected importance in Holmari beel while water temperature, pH, magnesium, hardness, dissolved organic matter, total dissolved solids and sulphate showed importance in Ghotonga beel.
While explaining limited influence of individual abiotic and lower cumulative influence of 17 abiotic variables on zooplankton assemblages (vide Canonical Correspondence Analysis), this study suggested that zooplankton taxa are rather generalists in terms of general abiotic factors, with factors associated with microhabitat being more important. The latter feature supported hypothesis of Sharma and Sharma3 vis-a-vis abiotic factors on Rotifera diversity in the floodplain lakes of northeast India.
To sum up, this study merits ecosystem diversity importance vis-à-vis quantitative dominance of the species rich zooplankton of Ghotonga beel and its sub-dominance of Bhereki and Holmari beels with quantitative importance of Rotifera > Rhizopoda in Bhereki and Ghotonga beels, and of Rhizopoda > Rotifera in Holmari beel. The richness, abundance and species diversity of zooplankton followed no definite pattern of monthly variations. The results affirmed higher species diversity, higher evenness and lower dominance of zooplankton and are characterized by lower densities of a majority of species. The limited individual influence and low cumulative influence (vide CCA) of seventeen abiotic factors on zooplankton assemblages of the Majuli beels affirmed that zooplankton taxa are rather generalists in terms of general abiotic factors and thus suggested importance of factors associated with microhabitat variations.
We thank the Head, Department of Zoology, NEHU, Shillong, for laboratory facilities. The field-work for this study was partially supported under the ‘UPE (Biosciences) Program’ of North-Eastern Hill University, Shillong.
None.

©2017 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.