Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 1
1North Africa Sea Turtles Network (NASTNet), Egypt
2Nature Conservation Sector, Egyptian Environmental Affairs agency, Egypt
3Red Sea protectorates, Nature Conservation Sector, Egyptian Environmental Affairs agency, Egypt
4Genetic Engineering Research Center, Department of Genetics, Faculty of Agriculture-Cairo University, Egypt
Correspondence: Nahla M. Naguib, North Africa Sea Turtles Network (NASTNet), Egypt
Received: April 02, 2024 | Published: April 25, 2024
Citation: Naguib NM, Salama A, Elsadek IM, et al. Using ISSR markers to detect genetic variation for marine turtles populations in Egypt loggerhead (Caretta caretta) and green turtles (Chelonia mydas). J Aquac Mar Biol. 2024;13(1):37-42. DOI: 10.15406/jamb.2024.13.00394
In Egypt, along the Mediterranean coast, the loggerhead (Caretta caretta) and green (Chelonia mydas) come during nesting season to lay their eggs or for food with a rare occurrence for the leatherback turtle (Dermochelys coriacea). In the Egyptian Red Sea there are five species that come to visit, the most common to observe are hawksbill (Eretmochelys imbricata) and green (Chelonia mydas). The goal of this study is to conduct some genetic and ecological analyses a) to generate new markers that include other segments of marine turtles’ genome, as a tool to investigate new areas of variation/polymorphism between, among, and within populations and individuals; b) determine genetic diversity between and within the Mediterranean and the Red Sea populations using (individuals from the same and different habitats using ISSR); and c) determine allele frequency. At the ecological level, this study aims to assess sea turtles' habitat and population distribution among different habitats in Egypt. Throughout the duration of this study, it was noted that there was a decrease in nesting areas attributable to the loss and fragmentation of habitats. Although there are some areas with no significant nesting prosperities it considered significant areas for foraging and highly important as migratory corridors, especially in the Mediterranean. The massive urbanization and unplanned touristic developments affect sea turtle habitat over the Egyptian Mediterranean coast. The Mediterranean coast showed demographic changes in the coastal regions. On the other hand, areas could still be valued with significant nesting areas for sea turtles, but an intense and regular monitoring programme should be established. The Red Sea needs a regular monitoring programme for better management conservation measures to be adopted. The polymorphism percentage resulting from ISSR marker was 45 and 36 for both loggerhead and green turtle populations in the Mediterranean, respectively; and 69 for the green turtle populations in the Red Sea.
Keywords: sea turtle, loggerhead, green, Caretta caretta, Chelonia mydas, ISSR
Study area
Fieldwork was carried-out from 2016 to 2019 and is divided into two main sections, a) during nesting seasons of Chelonia mydas (green sea turtle) and Caretta caretta (loggerhead sea turtle) from 2016 to 2019 (Figure 1) on both Mediterranean and Red Sea coast in Egypt, b) off nesting seasons survey and stranded individuals. These locations represent most known nesting sites, sites that were predicted to be suitable as nesting, foraging, and wintering areas.
Monitoring methodology
Monitoring was conducted according to UNEP guidelines (UNEP(DEPI)/MED WG.431/ Inf.4), through night and day patrols where night patrols allow direct observation of nesting female sea turtles while day or morning patrols to observe new tracks and missing nests, from 3 to 5 days for each location (Figure 1). This was to fulfil the following: a) beach assessment, b) identification of regular nesting sites and population distribution through the direct observation of nesting females, observed nesting sites, Observed Clutches Frequency (OCF) (numbers of nests/beach/season), and Stranded individuals, and c) size measurement of individuals, standard curved carapace length (CCL) and curved carapace width (CCW). Field work was targeted nesting females and stranded individuals.
Samples collection and DNA extraction
Skin, buccal swab and blood samples were collected from 26 individuals according to Naguib et al.1
DNA was extracted by using BioBasicEZ-10 Spin Column Genomic DNA Minipreps kit (blood) and Applied Biosystems™ MagMAX™ DNA Multi-Sample Ultra Kit for both tissue and buccal swab samples. DNA was collected and stored at −70°C until use. One percent agarose gel electrophoresis and spectrophotometer were used to determine the quality and quantity of DNA. DNA extraction and molecular analyses were performed in the Genetic Engineering Research Center, Faculty of Agriculture Cairo University.
Inter-simple sequence repeat (ISSR)
Inter-Simple Sequence Repeat (ISSR) PCR amplification technique was processed according to Baruah et al.2 using 5 primers (Table 1). The PCR reaction was performed in 20μl reaction mixture containing 1 μl (»25 ng) DNA; 1μl of designated primer; 10μl GeneDireX ready-to-use PCR reaction mixture containing Taq DNA polymerase, PCR reaction buffers, and dNTPs; and 8μl sterilized dH2O. PCR reaction optimized at 5 min of initial denaturation at 94°C, then 35 cycles at annealing temperature shown in Table 1 for 45 s, followed by 2 min at 72°C, with a final extension step of 5 min at 72°C. PCR product resolved on 1.5 – 2% agarose gels with TAE buffer containing ethidium bromide. LabImage 1D Version 7.1.3 Core 4.2.3 (Kapelan Bio-Imaging, Leipzig, Germany) software was used for band-scoring pattern analyses. The Systat ver. 7 computer programs were used to measure the pairwise differences matrix and plot the dendrogram among 26 individuals. Cluster analysis was based on similarity matrices obtained using the arithmetic average for dendrogram estimation with the unweighted pair-group method (UPGMA). Allele frequency analyzed by Cervus 3.0.3 - © Copyright Tristan Marshall 1998-2007, Distributed by Field Genetics Ltd - www.fieldgenetics.com - Licensed for non-commercial use only.
|
Primer |
Sequence |
Tm |
Ta |
|
P1 |
5`-(GA)8YT-3` |
48 |
42 |
|
P4 |
5`-(TC)8YG-3` |
52 |
45-47 |
|
P5 |
5`-(TC)8HT-3` |
50 |
43-45 |
|
P6 |
5`-(TC)8BG-3` |
52 |
45-47 |
|
P10 |
5`-(AG)8YT-3` |
47 |
42 |
Table 1 Primers designed for ISSR
H= non –G; V= non –T; B= non –A; Y= pyrimidine; R= purine
Molecular ecology is a science related to conservation genetics, concerning applying molecular genetics tools and data to study ecological issues related to evolution, behaviour, and ecology. Molecular ecology aims to improve measures taken for biodiversity assessment and conservation. Through molecular ecology genetic diversity can be identified within populations for better conservation measures.3
In achieving goals of the current study, the results obtained were as follows:
Ecological studies
Beach assessment has been performed through direct observation of the selected locations under this study. According to these observations, the type of each area was identified.
Along the Mediterranean coast in the selected study areas, habitat suffers from fragmentation due to activities associated with (a) the coastal development that is taken over most of the coastal areas either for recreational/tourist activities and/or (b) urban development; and (c) interaction with fisheries. Most of the selected locations were identified as important areas for foraging than nesting habitats, as in Table 2. While the Red Sea locations, selected in this study, maintain their properties as nesting and foraging areas sites.
|
|
Site |
Type of area |
|
|
Mediterranean |
Lake Bardawil (shoreline sea-side) |
Nesting |
Frequent |
|
Foraging |
Frequent |
||
|
Port Said |
Foraging |
Frequent |
|
|
Burullus |
Foraging |
Frequent |
|
|
Dammitta |
Foraging |
Frequent |
|
|
Alexandria |
Foraging |
Frequent |
|
|
El-Alamine |
Nesting |
N/A |
|
|
Foraging |
Frequent |
||
|
Marsa Matrouh |
Nesting |
N/A |
|
|
Foraging |
Frequent |
||
|
El-Salloum |
Nesting |
N/A |
|
|
Foraging |
Frequent |
||
|
Red Sea |
Ras Muhammed |
Nesting |
Frequent |
|
Zabargad |
Nesting |
Frequent |
|
|
Wadi El-gemal |
Nesting |
Frequent |
|
Table 2 Type of observed habitat
N/A: Not Applicable (need further study)
Through the direct observation of nesting females and observing nesting sites, the numbers of total observed clutches was 89 (Table 3, Figure 2) and the numbers of confirmed nests were 60 nests in total. The total numbers of individuals (alive and stranded) were taken into consideration for the identification of populations’ distribution to determine the significance of each selected study area for nesting foraging or both.
|
Location |
No. of Observed Clutches |
|||||
|
|
|
2016 |
2017 |
2018 |
2019 |
|
|
Ras Muhammed |
Qony bay |
15 |
10 |
N/D |
9 |
N N/D N |
|
Swaisy |
||||||
|
Turtle beach |
||||||
|
North Sinai |
|
N/D |
N/D |
N/D |
6 |
FW |
|
Wadi El-gemal |
Um Elabas |
N/D |
N/D |
N/D |
8 |
FW |
|
Tarfa |
||||||
|
Hankurab |
||||||
|
Zabargad |
|
40 |
N/D |
N/D |
N/D |
N |
|
Ashtoum |
|
- |
- |
- |
- |
FW |
|
Dammitta |
|
- |
- |
- |
- |
FW |
|
Alexandria |
|
- |
- |
- |
- |
FW |
|
Dabaa |
|
N/D |
N/D |
1 |
N/D |
SN |
Table 3 Clutches frequency (CF)
N, Nesting sites; N/D, Not Determined; FW, Foraging and Wintering sites; SN, Sporading Nest
As per Kaska et al.,4 Casale et al.,5 and Hochscheid et al.,6 the Egyptian Mediterranean coast might not be one of the important nesting areas in the Mediterranean, but it is known to be one of the most important areas as feeding ground and migratory corridors7 in the Mediterranean basin. The studied areas of the Egyptian Mediterranean coast showed habitat fragmentation in the most of north coast areas due to massive touristic development and unplanned urbanization over the past 40 years. That subsequently affects the suitability of the beaches to continue as nesting areas. However, due to the importance of the Egyptian Mediterranean coast as a migratory corridor and significant role conservation and management of sea turtles, even though high-stranding sea turtle individuals reported.
The size measurements were carried out for all individuals under study whether from nesting sea turtle females or stranded individuals. The curved carapace length (CCL) ranged from 86 to 94 for adults and 15 for juvenile loggerheads from 150 to 90 for adults and 45 for juvenile green sea turtles. The curved carapace width (CCW) ranged from 55 to 60 for adults and 9 for juvenile loggerhead from 100 to 87 for adults and 28 for juvenile green sea turtles.
In the agreement with Abdelwarith and Naguib8 and through the field observation and several surveys that were conducted with fishermen and local communities; there are no nesting activities in Ashtoum Elgamil protected area, Dammietta, El Burullus, and Alexandria. That indicated the effect of unplanned urbanization, changes in shore characteristics, and massive and unplanned touristic constructions and activities. With the sporading nests that were reported in Alalamine and with the suitability of the north coast (from Dabaa to El-Sallum) more monitoring activities need to be taken in place.
In agreement with Al Ameri et al.,9 Phillott et al.,10 and Mancini et al.,11,12 the studied areas in the Red Sea showed different pattern as they still keep their importance as nesting and foraging areas. The Zabargad Island is considered one of the most important nesting sites in Egypt and the Red Sea as well, where the estimated number of nests per year according to Mancini et al.12 »500 – 600 nests.
Wadi El-gemal protected area is also considered as one of the important places for the sea turtle nesting seasons in Egypt. There was annual monitoring programme till 2008 with the result from this study (8 nests discovered at the time of the study) compared to Phillott et al.10 and Mancini et al.11,12 (average of nests/year 19.3 nests) the same pattern of nests is still stood. Ras Bagdadi and Um El-Abas have been monitored annually from 2001 to 2008, with respectively an average of 19.3 and 16.3 nests on each site.
Ras Muhammed Protected areas through the time of the study especially Qony bay and Turtle Beach recognized by that average level of nests during nesting season but still need more study and a long-term monitoring programme should be applied.
Although climate changes have a role in changing behaviour of sea turtles either by affecting their nesting beaches or by changing their feeding ground, in the Red Sea the climate changes have a slight effect that cannot be observed.
Molecular techniques
The molecular markers used to determine genetic variation in marine turtles were to address relationship between foraging areas and rookery origins, and a) assessing population genetic structure,13–21 b) studying origin of populations and natal homing phenomenon,22–24 c) hybridization between two or more species, d) ability of female marine turtles to mate with more than one male is what so called multiple paternity phenomenon,25–27 e) studies related to temperature and sex ratio, how sex determination in sea turtles dependent on temperature not gametes (sex linked markers),28–30 conservation genetics studeis.25,31
Mitochondrial DNA, microsatellites and macro satellites, and nuclear DNA were extensively used to resolve these issues. Various molecular markers techniques such as microarrays, RAPDs, RFLPs, AFLPs and SNPs have been used for marine turtles’ research.25,32–36
All the used markers either nuclear or mtDNA were always used to determine the genetic variation and polymorphism between marine turtle populations, hence, expanding the positive impacts of conservation efforts towards determined measures.37 Several types of makers such as RFLP24 and AFLP34 identify diversity between examined populations toward better conservation measures and decisions.
The development of a set of SNP loci for marine turtles promises to be a rapid way of genotyping individuals,34 which can eliminate the variability of microsatellite genotypes due to differences in technology and scoring methods between laboratories.38 These methods can be applied to a broad range of taxa to aid in a variety of applications including ecological, behavioural, forensic, and population structure studies.
This study serves as initial experiment aimed at producing novel markers encompassing additional segments within the genome of marine turtles. This approach serves as a tool for exploring new realms of variation and polymorphism across populations and individuals.
Five primers have been used to detect polymorphism through ISSR as in Table 3. According to the ISSR banding pattern analyses (Table 4, Figure 3), in the Mediterranean populations, the polymorphism percentage for both loggerhead and green populations was 45% (25 polymorphic bands out of 56 scorable bands) and 36% (23 polymoporphic bands out of 64 scorable bands), respectively. The highest polymorphism detected by primer P4 for Loggerhead and P5 for Green populations, while the lowest polymorphism percentage detected by P1 for Loggerhead and P6 for Green populations.
|
Mediterranean |
||||||||
|
Loggerhead |
Green |
|||||||
|
Primers |
S.B. |
P.B. |
P% |
Amplicon size range (bp) |
S.B. |
P.B. |
P% |
Amplicon size range (bp) |
|
P1 |
19 |
1 |
5 |
180-513 |
18 |
5 |
28 |
146-562 |
|
P4 |
8 |
8 |
100 |
408-1078 |
12 |
4 |
33 |
162-1078 |
|
P5 |
7 |
6 |
86 |
332-843 |
6 |
6 |
100 |
424-1000 |
|
P6 |
13 |
5 |
38 |
198-1991 |
16 |
3 |
19 |
200-1405 |
|
P10 |
9 |
5 |
56 |
420-1661 |
12 |
5 |
42 |
470-1631 |
|
Total |
56 |
25 |
45 |
64 |
23 |
36 |
||
|
Red Sea (Green sea turtle) |
||||||||
|
Primer |
S.B. |
P.B. |
P% |
Amplicon size range (bp) |
||||
|
P1 |
9 |
9 |
100 |
220-1075 |
||||
|
P4 |
21 |
13 |
62 |
241-1452 |
||||
|
P5 |
17 |
11 |
65 |
312-1503 |
||||
|
P6 |
16 |
10 |
63 |
197-1488 |
||||
|
P10 |
9 |
7 |
78 |
384-1552 |
||||
|
Total |
72 |
50 |
69 |
Table 4 No. of polymorphic bands and polymorphism % by 5 ISSR primers for green and loggerhead sea turtles populations in the Mediterranean and the Red sea
S.B, Scorable band; P.B, Polymorphic band; P%: Polymorphism percentage.
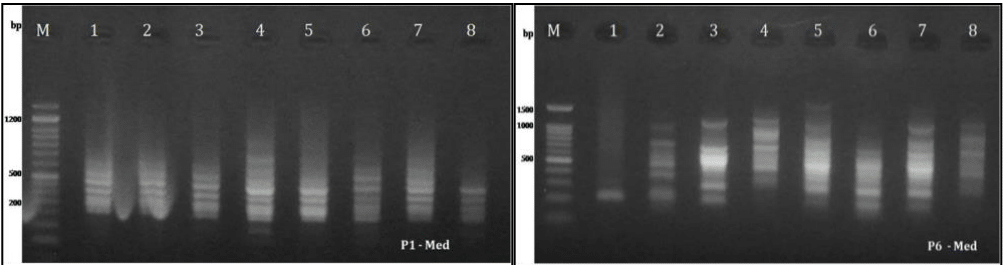
Figure 3 ISSR banding pattern of the Mediterranean populations (1-4 Chelonia mydas, 5-8 Caretta caretta) (M: Marker, bp: basepairs), 50bp and 100bp DNA Ladder © GeneDirex.
In the Red Sea population, the polymorphism percentage for green populations was 69% (50 polymorphic bands out of 72 scorable bands). The highest polymorphic bands detected by primer P4 with 13 polymorphic bands, while P10 recorded as the lowest with 7 polymorphic bands (Table 5, Figure 4).

Figure 4 ThreeISSR banding pattern of the Red Sea population the green turtle (Chelonia mydas) (1-17) and the logger head (Caretta caretta) (18) (M: Marker, bp: basepairs), 100bp DNA Ladder © GeneDirex.
The numbers of observed alleles, as represented in Table 5, were 27 for P1 and P5, 24 for P4, and 25 for P6 and P10. While the number of the effective alleles is 15.9059, 15.7209, 14.5376, 13.6566, and 13.2549 for P5 P10, P4, P3, and P6, respectively. The frequency for detected alleles ranged from 0.0192 to 0.1538 for P1, P5, and P6; from 0.0192 to 0.1346 for P4; and from 0.0192 to 0.1154 for P10.
|
Locus |
Na* |
Ne* |
I* |
|
P1 |
27.0000 |
13.6566 |
2.9559 |
|
P4 |
24.0000 |
14.5376 |
2.9078 |
|
P5 |
27.0000 |
15.9059 |
3.0400 |
|
P6 |
25.0000 |
13.2549 |
2.9067 |
|
P10 |
25.0000 |
15.7209 |
2.9678 |
|
Mean |
25.6000 |
14.6152 |
2.9556 |
|
St. Dev |
1.3416 |
1.1899 |
0.0547 |
Table 5 The number of observed and effective alleles
Na, Observed number of alleles; Ne, Effective number of alleles; I, Shannon's Information index
The examined individuals were divided into two major groups at the 0.56 similarity level; one group contained only the Mediterranean populations. The other group was divided into three groups at similarity level 0.38. The Mediterranean green turtle population, MG1, MG2, and MG4 were clustered in one group, as they are from adjacent locations Alexandria and El-Sahel while M3 from Port Said. The same goes for the Mediterranean loggerhead ML1, ML2, and ML4 with some exception for ML3. Also, there were individuals from the Red Sea green turtle population showed a distinguished relationship with each other; where there was close relationship among individuals RG2, RG3, RG4, RG5, RG8, RG9, RG10, RG12 from Zabargad Island, RG13, RG14, RG15, RG16 from Ras Muhammad Protectorate and RG17 from Hurghada. The ISSR clustered the examined populations within two species and separately grouped each population within the given index (Figure 5).

Figure 5 The Mediterranean and Red Sea populations for both Chelonia mydas and Caretta caretta by ISSR marker, ML, Mediterranean loggerhead; MG, Mediterranean green; R, Red Sea.
The genetic variation is crucial for the resilience and adaptation to the environmental changes. Therefore, there is a continuous need to reach for more molecular markers to assess variation and diversity between and within populations.
The ISSR markers were used to get more information on variation at the level of repetitive segments.39 Yan et al.,39 used ISSR markers to detect genetic variation between populations and Marwal and Gaur40 stated, ISSR combines merits of SSR and AFLPs to obtain additional information on genetic diversity, tagging gene tagging, genome mapping, and evolutionary biology.41 Therefore, using ISSR will aid in the discovery of additional segments along the sea turtle genome to address variation between and within studied populations addressing phylogeographical analyses appropriately.
Results obtained indicated that there were variations between Mediterranean and Red Sea populations with a high variation among individuals within the same population. There was significant polymorphism within the studied individuals/population.
According to the similarity level, the cluster tree showed how much the Mediterranean and Red Sea populations are distinguished from each other into 2 completely different clusters. Besides, similarity between individuals from Ras Muhammad PA, Hurghada, and some individuals from Zabargad showed distinguished and close relationships.
There is a decline in the number of nesting areas due to habitat loss and fragmentation. Although, there are some areas with no significant nesting prosperities, but it considered significant areas for foraging and highly important as migratory corridors, especially in Mediterranean. The massive urbanization and unplanned touristic developments affect the sea turtle habitat over the Egyptian Mediterranean coast. The Mediterranean coast showed demographic changes along the coastal regions. On the other hand, there are areas that still could be valued with significant nesting areas for sea turtles, but an intense and regular monitoring programme should be established. The Red Sea needs regular monitoring programme as well for better management conservation measures to be adopted.
Using ISSR molecular markers is a point for experiencing developing new techniques to generate more markers for assessing polymorphism, diversity, and genotyping of marine turtles. Between the studied populations, green turtle Red Sea populations showed the highest polymorphism percentage. According to cluster analysis and the phylogenetic tree, the populations from the Mediterranean have a different evolutionary fork than populations from Red Sea. More sites need to be monitored along Mediterranean to have a better insight into Mediterranean population structure.
None.
The authors declare that there are no conflicts of interest.

©2024 Naguib, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.