Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 4
1Department of Fisheries and Aquatic Science Cross River University of Technology Nigeria
2Department of Fisheries Livestock Production Technology Niger Delta University Nigeria
3Department of Fisheries and aquatic environment Rivers State University of Science and Technology Nigeria
Correspondence: Okey IB Department of Fisheries and Aquatic Science Cross River University of Technology Obubra Campus Cross River State Nigeria
Received: May 06, 2018 | Published: July 3, 2018
Citation: Okey IB, Keremah RI, Gabriel UU. The efficacy of clove ( Eugenia caryophyllata ) powder as anaesthesia on African catfishes ( Clarias gariepinus and Heterobranchus bidorsalis ) fingerlings. J Aquac Mar Biol. 2018;7(4):182?188. DOI: 10.15406/jamb.2018.07.00206
The efficacy of clove powder as anaesthetic agent on C. gariepinus and Heterobranchus bidorsalis fingerlings was investigated in this study and the water quality parameters of the test solutions also determined. The time of induction and recovery for various stages and concentrations was recorded in minutes using a stop watch. The concentrations used for the anaesthesia bioassay were 60, 70, 80, 100, 120, and 140mg/l. The water quality parameters examined after 30mins did not differ (P<0.05) from those of control hence may not have contributed to the observed behaviour of the test fish. Fingerlings exposed to 60 and 70mg/l had partial anaesthesia and did not achieved stage 4(deep anaesthesia) after 30mins. Those exposed to concentration above 70mg/l were completely immobilized (deep anaesthesia) within 3–10mins depending on concentration. The mean time under each concentration increased significantly (P<0.05) from stages 1-4. The various stages of induction and recovery of C. gariepinus were higher than those of H. bidorsalis except induction stages 1 and 3 and recovery stage 3. Increasing concentration proportionally decrease the induction time but increase the time required for full recovery. An ideal anaesthetic must have among other qualities quick induction and slightly longer recovery time to allow for varied manipulation as desirable. In this study clove powder at 120 and 140 mg/l induced deep anaesthesia in less than 5mins with a recovery time of 25–27mins, making it an ideal anaesthestic for African clarrid fingerlings.
Keywords: clove powder, african catfishes, fingerlings, induction, recovery
Aquaculture practices frequently exposed fish to a variety of acute stressor that have the potential to negatively affect the normal performance and survival of organism.1,2 Anaesthetic abolishes pain in fish and induces a calming effect, loss of equilibrium, mobility and consciousness.3,4 In fisheries research and aquacultural operations, anaesthetics are necessary to minimize stress and reduce physical injury of fish during various handling procedures and transportation.5,6 Anaesthetics have also been reported to minimize mortality and reduce susceptibility to pathogens and infections.7 Anaesthetics most commonly used by commercial fish culturist and biologist include metomidate, tricaine methane sulphonate (MS 222), etomidate, benzocaine- hydrochloride, phenoxyethanol and eugenol.8‒10 Some plant extract such as Derris (rotenone), tephrosia (tephrosin), Erythrophleum (alkaloid and tanin), Pyrus (tannin and saponin) and Tobacco (nicotine) have also been reported to have anaesthetic effects on fish.11‒13 Clove oil derived from clove plant Eugenia caryophyllata (4- allyl–methoxylphenol) is regarded as the best anaesthetics to various fishes.14,15
The desirable attributes of anaesthetics used for fin fish include, short induction and recovery time, non-toxicity to fish and humans, no lasting physiological effects, rapid clearance from the body, high solubility in fresh and salt water, availability and cost effectiveness.8,16 Sudagara et al.,16 stated that although clove oil is adjudged the best anaesthtics for aquaculture organism but not readily soluble in water, not easily available and costly. To solve this problem, biologists and aquaculturists have been searching for alternative anaesthetics that apart from been less toxic, efficacious and safe for humans, should be readily available and cheap.17,18
Clove powder is derived from the dry flower buds and stalks of clove plant E. caryophyllata. The efficacy of this powder has been evaluated on common Iranian fish Roach, Rutilus rutilus.16 Clove powder is readily available, highly soluble in water and cheap. In Nigeria clove products are sold in most herbal shops and markets use primarily for flavouring food (spice) and supplement for topical treatment of tooth ache, common cold, cough and inflammation of the mouth and throat.19 In cross river, clove flower buds are commonly known as “zobo” spice sold in most markets across the state.
The African catfishes, Clarias gariepinus and Heterobranchus bidorsalis constitute the largest group of cultured species after carp, salmonids and tilapia, and they grow well under various culture systems of the world.20 Their culture is becoming more popular among fish farmers in Cross River, where they are being transported from hatcheries sites to grow out ponds research labouratories. According to Sudagara et al.,16 clove powder is highly soluble in water, cheap and effective anaesthetic to Roach with reversible damage to damage to bloodstream. Similar observation was reported by Okey et al.,21 for hybrid catfish on clove powder. The aim of the study is the determine the efficacy and effective concentration of clove powder on the two species of African catfishes C. gariepinus and H. bidorsalis fingerlings.
Dry E. caryophyllata flower buds were procured from a herbal shop in watt market Calabar, Calabar South Local Government Area of Cross River State, Nigeria. The materials were identified at the Department of Botany University of Calabar (UNICAL). They were taken to the Fisheries Laboratory Cross River University of Technology (CRUTECH,) Obubra Campus. The flower buds were sundried for 30 min and then pulverized with a sterile manual blender and sieved with 100 micron net to obtain a fine powder. The powder was put in an airtight container and stored in a dry place prior to commencement of the experiment. Two hundred each of apparently healthy fingerlings of C. gariepinus (3.26g±2.30SD; 8.34cm±1.82SD) and H. bidorsalis (2.89g± 1.80SD; 7.55cm ±1.04SD) were procured from UNICAL fish Farm, Calabar and acclimated for 14 days at the Fisheries Wet Labouratory, Cross River University of Technology (CRUTECH) Obubra, Nigeria. No mortality was recorded during the period of acclimation and feeding was discontinued 12hours before and during the experiment to minimize contamination of the test medium. A stock solution of clove power with a concentration of 200mg/L was prepared by dissolved 2g of clove powder into 10L of borehole water. Exposure concentrations of clove powder were 60, 70, 80, 100, 120 and 140mg/l, respectively. Thirty six glass aquaria were clean and randomly labeled and each filled with water to the 15litres mark for induction test and 20L mark for recovery. The different concentrations were prepared by serial dilution of the stock solution and water added to make up to 20L that gave the desired test concentrations. The mixture was stirred with a glass rod for homogenous mixing. Each concentration was stock with 10 fingerlings each in triplicate and monitored for the onset of induction (anaesthesia) for 30 min as periods greater than this were considered not ideal and impractical for routine fish handling procedures.14,22 The test fish were monitored following the various stages of induction and recovery time using a stop watch (Table 1). Any of the test fish that lost balance and ceased respiratory movements of the opercula (deep anaesthesia, stage 4) was removed immediately and transferred to about 20L of clove powder free water. The time for the fish to enter the desirable anaesthesia level (induction) and that which is required for an anesthetized fish to regain equilibrium and began active swimming (recovery time) were recorded at each stage.
During the study, the following water quality parameters were monitored in the experimental and control tanks: temperature, pH dissolved oxygen, alkalinity, conductivity and hardness. The water temperature was measured using the mercury in glass thermometer and recorded in 0C. Conductivity was with conductivity meter (PACM 35 Model) and pH meter (Model 3015 Jenway) for pH. Dissolved oxygen was measured with a digital oxygen meter. Alkalinity was determined by standard method.24 Total hardness was determined by ethylene diamine- tetra acetic acid (EDTA) titration method. Data obtain from the various stages of induction (anaesthesia) and recovery time were subjected to various statistical tools. Descriptive statistic was used to obtain the means and standard error (Mean±S.E). Differences among mean time for different dosage to achieve various stages of anesthesia and recovery time on concentration were subjected to one way ANOVA using SPSS 18.0 version. Differences in means were separated using Tukey–Honest Significant Different (T-HSD) test and F-test for significant level at (p<0.05) between the treatments.25,26
Water quality parameters
The result of the water quality parameters as shown in Table 2 indicates that their mean values did not differ (p>0.05) from those of the control. The mean values of the various parameters decreased with increasing concentrations as follows, temperature (28.13-26.88oC), dissolved oxygen (4.74–4.12mg/l), pH(6.78–6.77) and hardness (39.32–38.80mg/l CaCO3). Conductivity (166.07-167.05µScm/l) and alkalinity (37-41-38.51mg/l) had a slight increase above those of control at 140mg/l of clove powder after 30mins of exposure.
Induction and recovery of gariepinus and H. bidorsalis fingerling from clove powder anaesthetic
The C. gariepinus fingerlings exposed to concentrations below 80mg/l did not reach stage 4 (complete immobilization) but with partial loss of equilibrium (stages 1–3) in 6–13mins. At 80mg/l, C. gariepinus fingerlings lost equilibrium in 6-9 mins and completely immobilized in 12 mins and begins recovery in 7-9mins. At 120 and 140 mg/l fish were completely anaesthetized (stage 4) within 2-5mins and regained equilibrium between 10–27mins. No mortality resulted from anaesthesia within 60-120mg/l of clove powder after 30 mins of exposure. The mean induction time under each concentration increased significantly (P<0.05) from stages 1–4. At the highest concentration (140mg/l), the induction stages were not significant (P>0.05) except at stage 4 (Table 3). The ANOVA indicated that powder caused significant changes ((P<0.001) on all the stages of induction and recovery.
The anaesthesia and recovery time of H. bidorsalis from clove powder were similar to those of C. gariepinus fingerlings in which increasing concentrations of clove powder proportionally decreased the time required for induction. No complete immobilization was achieved between 60–70mg/l of the anaesthetic. The time required to attained partial anaesthesia at stage 3 (19.00 and 10.00mins) was significantly higher than that of stage 2(10.88 and 7.73) for 60 and 70mg/l respectively. At 120–140mg/l, the time to achieved complete immobilization was 4.93 and 2.33mins and completely regained equilibrium in 16.80 and 25.20mins respectively. At 140mg/l stages induction stages 1 and 2 were not observed and the time to achieved stages 3 (1.20min) and 4 (2.33min) were not significant (P<0.05). The recovering time at stages 3 was higher (p<0.05) than those of stages 1 and 2 (Table 4). The ANOVA showed that clove caused highly significant (P<0.001) on the stages of induction and recovery.
The induction stages of the fingerlings at the various levels of concentrations were not significant (P>0.05) except at 60 and 100mg/l where the time in C. gariepinus was higher (Table 5). The mean values of the various stages of induction and recovery showed that, stages 2, 4 (deep anaesthesia) and recovery stage 1 were significantly higher (P<0.05) in C. gariepinus. The mean time to regain complete equilibrium (recovery 3) in the both species was not significant (P>0.05) (Figure 1). The interaction of species and concentrations also had significant changes (P<0.01) on all the stages of induction and recovery except at induction stage 1 and recovery stage 3, which was not significantly ( P>0.05) different.
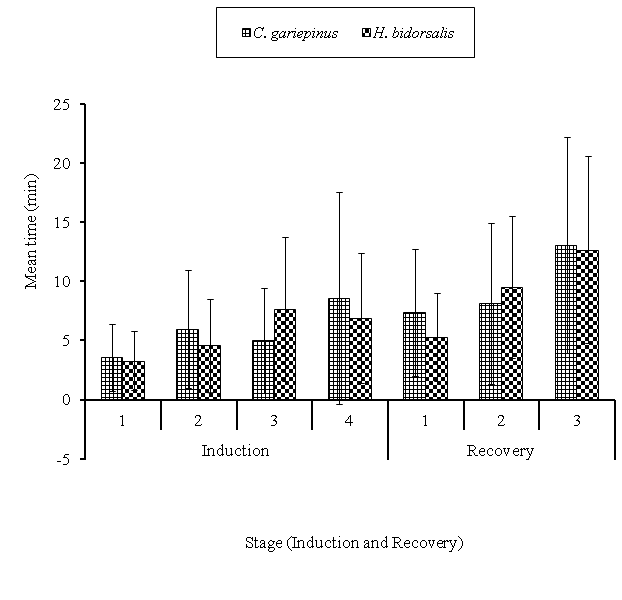
Figure 1Comparism of the various stages of induction and recovery time (min.) of C. gariepinusand H. bidosarlis fingerlings exposed to clove powder anaesthetic for 30 min. Bars ±S.D Mean with different letters differs significantly (P <0.05;HSD).
Stages |
Anaesthesia(induction) |
Stages |
Recovery |
Behavior of the fish |
Behavior of the fish |
||
I |
Acceleration of the opercular movements, increased respiratory activity, accompanied by uncoordinated locomotion. |
I |
Body immobilized but opercular movements just starting and weak, uncoordinated locomotion |
II |
Sporadic loss of equilibrium, difficulty maintaining position while at rest, high reaction to external stimuli. |
II |
Regular opercular movements and gross body movements beginning. |
III |
Complete loss of equilibrium; inability to regain upright position. |
III |
Equilibrium regained, normal swimming and pre-anaesthetic appearance. |
IV |
No reaction to handling or a sharp prod in the peduncle. |
Table 1 Stages of anaesthesia (Induction) and recovery
Source: Iwama et al.,23)
Conc. |
Parameter |
|||||
Dissolved Oxygen |
Temperature |
pH |
Conductivity |
Alkalinity |
Hardness (mg/lCaCO3) |
|
0 |
4.74±0.27a |
28.13±1.07a |
6.78±0.28a |
166.07±2.04a |
37.41±0.45a |
39.32±0.81a |
60 |
4.29±0.23a |
27.53±1.18a |
6.64±0.29a |
167.17±1.47a |
37.91±0.49a |
38.40±0.36a |
70 |
4.37±0.35a |
26.87±1.25a |
6.83±0.27a |
166.51±1.31a |
38.18±1.96a |
37.61±0.58a |
80 |
4.48±0.12a |
27.37±0.78a |
6.79±0.41a |
165.96±1.32a |
40.76±2.69a |
38.33±0.54a |
100 |
4.38±0.36a |
26.67±1.02a |
6.79±0.16a |
166.79±2.14a |
36.75±0.85a |
38.72±0.83a |
120 |
4.38±0.38a |
27.74±0.91a |
6.72±0.19a |
167.23±2.50a |
38.67±1.05a |
38.26±0.33a |
140 |
4.12±0.12a |
26.88±0.19a |
6.77±0.22a |
167.05±2.71a |
38.51±0.47a |
38.80±0.85a |
Mean with the same superscript under the same parameter are not significantly different at p< 0.05 |
||||||
Table 2 Water Quality Parameters in Experimental Tanks of African catfishes fingerlings exposed to clove powder for 30 mins
Conc. |
Stages of induction(min) |
Stages of recovery(min) |
|||||
I |
II |
III |
IV |
I |
II |
III |
|
60 |
7.73±0.24b |
14.73±0.47a |
0.00± 0.00c |
0.00± 0.00c |
0.00±00a |
0.00± 0.00a |
0.00±0.00a |
70 |
6.40±0.29c |
9.13± 0.43b |
12.07±0.41a |
0.00± 0.00d |
0.00±00a |
0.00± 0.00a |
0.00±0.00a |
80 |
3.60±0.20d |
5.73± 0.63c |
8.53± 0.63b |
11.20±0.72a |
6.47± 0.18b |
5.20± 0.73b |
8.47± 3.74a |
100 |
2.60±0.20d |
4.33± 0.13c |
5.73± 0.18b |
7.00± 0.35a |
8.33±0.24b |
8.60± 0.35b |
15.43±0.47a |
120 |
1.00±0.00c |
1.67± 0.07c |
2.73± 0.24b |
4.93± 0.13a |
9.90± 0.21c |
13.03±0.94b |
17.87± .22a |
140 |
0.00±0.00b |
0.20± 0.11b |
0.80± 0.12b |
2.13± 0.67a |
16.17±0.42c |
19.46±1.18b |
27.12±0.98a |
Means with the same superscript under each of the concentration are not significant (P>0.05) |
|||||||
Table 3 Amplitude in mill volts of the Lead-1 of electrocardiography in sheep
Con |
Stages of induction (min) |
Stages of recovery (min) |
|||||
I |
II |
III |
IV |
I |
II |
III |
|
60 |
6.47±0.27c |
10.88±0.27b |
19.00±1.50a |
0.00± 0.00d |
0.00±0.00a |
0.00± 0.00a |
0.00± 0.00a |
70 |
4.63±0.09c |
7.73± 0.18b |
10.00±0.12a |
0.00± 0.00d |
0.00±0.00a |
0.00± 0.00a |
0.00± 0.00a |
80 |
3.20±0.12d |
5.27± 0.13c |
7.87± 0.13b |
10.73±0.37a |
4.47±0.18c |
8.53± 0.18b |
12.17±0.45a |
100 |
1.20±0.12c |
2.33± 0.24c |
5.00± 0.20b |
7.60± 0.23a |
5.53±0.27c |
10.60±0.35b |
13.00±0.15a |
120 |
0.00±0.00c |
1.27± 0.07b |
2.93± 0.07b |
4.93± 0.47a |
6.57±0.32c |
13.80±0.31b |
16.80±0.35a |
140 |
0.00±0.00b |
0.00± 0.00b |
1.20± 0.12a |
2.33± 0.07a |
11.87±.24c |
18.40±0.49b |
25.20±0.93a |
Means with the same superscript under each of the concentration are not significant(P>0.05) |
|||||||
Table 4 The mean time(min) of induction and recovery of H. bidorsalis fingerlings from clove powder anaesthetic
Conc. (mg/l) |
Stage of induction (min) |
Stage of recovery (min) |
||||||||||||
1 |
2 |
3 |
4 |
1 |
2 |
3 |
||||||||
|
C.g |
H.b |
C.g |
H.b |
C.g |
H.b |
C.g |
H.b |
C.g |
H.b |
C.g |
H.b |
C.g |
H.b |
60 |
7.73 |
6.47 |
14.73 |
10.88 |
0.00 |
19.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
70 |
6.40 |
4.63 |
9.13 |
7.73 |
12.07 |
10.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
80 |
3.60 |
3.20 |
5.73 |
5.27 |
8.53 |
7.87 |
11.20 |
10.73 |
6.47 |
4.47 |
4.20 |
8.53 |
8.47 |
12.17 |
100 |
2.60 |
1.20 |
4.33 |
2.33 |
5.73 |
5.00 |
7.00 |
7.60 |
8.33 |
5.53 |
8.60 |
10.60 |
15.43 |
13.00 |
120 |
1.00 |
0.00 |
1.67 |
1.27 |
2.73 |
2.93 |
4.93 |
4.93 |
9.90 |
6.57 |
13.03 |
13.80 |
17.87 |
16.80 |
140 |
0.00 |
0.00 |
0.20 |
0.00 |
0.80 |
1.20 |
2.13 |
2.33 |
16.17 |
11.87 |
19.46 |
18.40 |
27.12 |
25.20 |
Means with the same superscript under each of the variables are not significant (P>0.05), C. gariepinus(C. g), H. bidorsalis(H.b) |
Table 5 Comparative mean values of various stages of induction and recovery of C. gariepinus and H. bidorsalis fingerlings on the various levels of clove powder
The time to recover from anaesthesia is dependent on concentration and induction time.38 The higher the concentration, the lower the time of induction (anaesthesia) and the longer the recovery time. Similar observation was also recorded in this study. For instance, (less than 9mins) and (more than 27mins) were required for fingerlings of the clarrids to regain full recovery at 80mg/l and 140mg/l of clove powder respectively. The recovery time was shorter when lower concentrations of the clove powder were used. These observations followed the same pattern as those described for clove oil by Hamackova et al.,32 in tench (Tinca tinca), Velisek et al.,40 in common carp (C. carpio) and Okey et al.21 in Afircan catfish hybrid. When the concentration of clove powder was increased, the time to loosing equilibrium, as well as reaching stage 4 (complete immobilization) declines. Other authors using clove oil anaesthesia on different fish species such as Keene et al.,41 Hoskonen and Pirhonen,47 Zaikov et al.,37 established similar inverse proportional dependencies between recovery time and concentrations. However, the time to regain full recovery from anaesthesia induced by clove powder on the fingerlings varies with the species. The fingerlings of C. gariepinus at 140mg/l achieved deep anaesthesia in 2.13min and recover at 27.12min whereas H. bidorsalis had the same induction time (2.33min), but a lower recovery time of 25.20min. similar result was demonstrated by Soto and Burhanuddin48 and Anderson et al.,49 who used clove oil (33-120mg/1) and reported recovery time of 150sec for Siganus lineatus and 190 sec for rainbow trout respectively. Several studies involving the clarrids confirm the above observation.13,50
Studies involving other botanicals used as anaesthetics show that clariid had higher recovery time under clove powder anaesthesia than others which according to Keene et al.41 is needed for prolong surgical manipulation of the fish. According to Adebayo et al.13, the effective concentration of avocado pear leaf extracts that completely anaesthetized C. gariepinus broodstooks was 190ml/l with a recovery time of 12mins. Ayuba and Ofejukwu50 reported that C. gariepinus fingerlings were anaesthetized in 10min and recovers within 2 min of immersion in freshwater after exposure to 2.0g/l of dried seed extract of toloache plant, D. innoxia. The essential oil of L. alba was used at concentrations of 100 to 500mg/l to induce anaesthesia (Stage 4) in silver catfish in 3-6mins and recover within 6-12mins.51 Menthol, the main component of the essential oil of plants from genus Mentha, at concentrations of 100–200mg/l induced deep anaesthesia in tambaqui after 1–2min and with recovery time of 5–12min.52 The recovery time recorded for clarrids in this study were slightly higher than those recommended for some European and Asian fishes. This implies that some water quality especially temperature can affect the rate of biodegradation and excretion of the toxicant from the blood streams via the gills hence affecting the recovery time.
Physiological responses in fish to anaesthetics are different. Kucuk2 reported that the gills area, body weight, species and metabolic rate have effects on the rate of anaesthetics absorption and induction (anaesthesia). This may have accounted for the slight differences in the induction and recovery time in the various species of clariids recorded in this study. Generally, an ideal anaesthetic ought to induce anesthesia quickly in less than 3 min, permit a fast recovery in 5min or less, produce no poison to fish, cause no hazard to human and be inexpensive.4 The result from this study indicated that clove powder at the concentrations of 80-140mg/l induced deep anaesthesia between 3-10min. with a recovery time of between 8–27mins making it an ideal anaesthetic for clariids. However, quick induction and a slightly longer recovery time would allow for varied manipulations as desirable, hence clove powder is an ideal anaesthetics for the African clarrid fingerlings.
We express sincere thanks to the Head Department of Fisheries Aquatic Science, CRUTECH and staff of RSUST for providing the necessary facilities, suggestions and guidance toward the accomplishment of this research.
The author declares that there is no conflict of interest.

©2018 Okey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.