Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 1
1Aquaculture Research Center, Portuguese Institute for the Ocean and Atmosphere, Olhão, Portugal
2Divisão de Aquacultura, Valorização e Bioprospeção, Portuguese Institute fot the Ocean and Atmosphere, Lisboa, Portugal
3SPAROS I&D Nutrition in Aquaculture, Olhão, Portugal
Correspondence: João Araújo, Aquaculture Research Center, Portuguese Institute for the Ocean and Atmosphere, Olhão, Portugal
Received: January 24, 2023 | Published: February 2, 2023
Citation: Araújo J, Loureiro P, Candeias-Mendes A, et al. The effect of a formulated feed on the body growth and gonads quality of purple sea urchin (Paracentrotus lividus) aquaculture produced. J Aquac Mar Biol. 2023;12(1):11-18 DOI: 10.15406/jamb.2023.12.00351
Sea urchin gonads are a prized gourmet product in many areas of the globe, and wild populations are threatened by increasing demand, making this species a very desirable product in aquaculture. Due to the unique needs of each stage of the life cycle, a viable food protocol that seeks to satisfy each stage while optimizing production profitability is required. The objective of this work was to test a formulated feed for sea urchins, through the analysis of somatic growth, gonad development and their fatty acid profile. These results were compared with sea urchins fed natural food (Ulva spp.) At the end of the trial Paracentrotus lividus juveniles fed inert diet had higher total wet weight gain, total specific growth rate, and gonadosomatic index than those fed with macroalgae. However, histological observations did not reveal differences in the maturation state of the gonads. In comparison to sea urchins fed fresh Ulva spp., inert diet showed higher total wet weight gain and higher total specific growth rate. Fatty acid contents of the gonads of each treatment were found to be similar. The DHA levels in the gonads of sea-urchins fed the formulated feed were significantly higher. It was possible to record the biosynthesis activity of some fatty acids such as 20:4 ω 6, 16:4 ω 3, and 20:3 ω 3. It was found that the presence of some essential fatty acids, such as DHA and EPA, in the gonads is only dependent on the food profile. The addition of a DHA-rich ingredient in the formulated feed may have been preponderant in the constitution and development of the sea-urchins’ gonads.
Keywords: sea-urchin, nutrition, Paracentrotus lividus, formulated feed, aquaculture, fatty acid
The purple sea urchin, Paracentrotus lividus, like other species of sea urchin, is of great economic interest, as its gonads are considered a delicacy.11,2 In certain countries, such as Japan, the sea urchin gonads, also known as “uni”, are fullypart of the local culture and gastronomy. With nearly 126 million inhabitants, Japan is the largest consumer of sea urchins in the world: it is estimated that 80-90% of the world's sea urchin production is consumed there (Sun and Chiang 2015). Meanwhile, Chile, with about 6,435 km of coastline, is the largest supplier of sea urchins, with more than 55,000 tons per year.1 In Europe, France, Spain, and Italy are the main consuming countries for sea urchin gonads, where their demand has increased considerably in recent decades.3 As the human body cannot synthetize certain essential fatty acids (EFAs), such as some n-3 and n-6 PUFAs, they must be consumed directly in the diet. This lack of PUFAs can be fulfilled with seafood, like sea urchins, as seafood in general is known to be an important source of those polyunsaturated fatty acids.4 As demand continued to increase and the capture of wild sea urchins was no longer ecologically sustainable, interest in echinoculture emerged.5–9 In fact, echinoculture, in addition to responding to the demand increase, has helped to relieve pressure from fishing in wild populations.10 One of the main obstacles encountered in the production of sea urchins are related to feed: difficulty in finding the balance between cost and quality of feed, limited growth due to inadequate diets and poorly optimized and efficient feeding regimes. The sea urchins’ commercial value can also be affected as certain diets can degrade the nutritional and organoleptic characteristics of the gonads.2,10,11 The use of macrophytes to feed sea urchins in an echinoculture has several disadvantages.10 The costs associated with transporting the seaweed and storing it are very high and, in a large-scale echinoculture, the amount of macroalga needed to feed sea urchins represent a very high investment because it requires large spaces to be stored.10 In addition to the economic aspect, their availability and nutritional characteristics vary depending on the season and location as shown in some studies.7,10–13 The negative aspects in the use of macroalgae in the feeding of sea urchins in aquaculture led to an increase in scientific research focused on their feeding, whose work found a common solution: the development of formulated diets.2,9 In other hand, inert diets are easier to store, can be kept at room temperature and allow greater feed control because the nutritional characteristics of the feed are known and invariable throughout the year.13 Several studies have also shown a greater somatic and gonadal growth in sea urchins when fed with inert diets.5,12
The aim of the present study was to determine the effects of a new formulated feed on somatic and gonadal growth of P. lividus, as well as studying the effect of formulated feed on the gonad’s fatty acid profile. For the formulation of this inert food, the main raw material used was macroalgae co-produced in local fish farms in order to avoid the need for harvesting in the wild and thus reduce the impact of the manufacture of this food on the marine environment.
Diet formulation
The inert formulated feed was developed and manufactured by the SPAROS I&D (Olhão, Portugal). Specially designed for equinoculture, they had a disc shape, incorporated Ulva spp. (to enhance the acceptance of sea urchins) and microalgae biomass (Schizochytrium sp.) for DHA (Docosahexaenoic acid) enrichment. This food also contains 20% of Ascophyllum nodosum, a kelp macroalgae widely used in aquaculture feed, rich in minerals, vitamins and trace elements, improving growth performance, immuno-stimulating and diseases resistance in many marine species.14 (Table 1)
Ingredients (%) |
Formulated feed |
Fish gelatine |
5 |
Macroalgae (Ascophyllum nodosum) |
20 |
Macroalgae (Ulva spp. supplied by IPMA) |
20 |
Wheat gluten |
7.5 |
Corn gluten meal |
17 |
Wheat meal |
10 |
Potato starch (gelatinised) |
0 |
Sorbitol |
0 |
Vitamin and mineral premix |
2 |
Antioxidant |
0.4 |
Monocalcium phosphate |
3 |
Calcium carbonate |
5 |
Binder (sodium alginate) |
0 |
Beta-carotene 10% |
0.5 |
Algae biomass (Schizochytrium 16% DHA) |
9.6 |
Table 1 Ingredients of the formulated feed
The control diet consisted of Ulva spp., a macroalgae produced naturally in the EPPO settling tank. In order to guarantee the same nutritional quality throughout the trial, a single collection of this alga was made at the beginning of the trial, with the total biomass being divided into doses and kept in plastic bags at a temperature of -20ºC. The choice of this macroalgae as a control is due to the natural abundance of this genus in this region of Portugal, growing easily in earth ponds used in fish farming. Furthermore, macroalgae of the genus Ulva are part of the natural diet of the sea urchin P. lividus in southern Portugal,15 and their application in aquaculture of these echinoderms has already been studied in previous works.8,12
Proximal analysis of both diets (Table 2) was carried with analytical duplicates and following the methodology described by Matias et.al. and AOAC.16,17 Total ash content was determined by combustion (550 °C during 6h) in a muffle furnace (Nabertherm L9/11/B170, Germany); crude protein ( ) by a flash combustion technique followed by a gas chromatographic separation and thermal conductivity detection with a Leco N Analyzer (Model FP-528, Leco Corporation, USA); crude lipid by petroleum ether extraction (40-60 °C) using a Soxtec™ 2055 Fat Extraction System (Foss, Denmark), with prior acid hydrolysis with 8.3 M HCl; gross energy in an adiabatic bomb calorimeter (Werke C2000, IKA, Germany).
|
Formulated feed |
Control (Ulva spp.) |
Protein (%) |
32.62 |
22.13 |
Fat (%) |
5.19 |
1.63 |
Gross energy (kJ g-1) |
15.75 |
11.03 |
Ash (%) |
21 |
11.03 |
Table 2 Proximal composition of the two diets (% dry matter)
Feeding trial
For this work, juvenile sea urchins produced at the Aquaculture Research Station (EPPO) of the Portuguese Institute for the Sea and Atmosphere (IPMA) were used. The mean initial weight was 12.95 (±3.36) and the test diameter was 3.73 (±0.39). Two experimental groups were created whose difference was based on the diet provided. The experimental group was fed the formulated feed) and the control group was fed the frozen macroalgae Ulva spp. For each experimental group, 3 replicates were established, each with a Ni of 50 sea urchins. Each replicate group (Ni=50) was placed in a Hexcyl® basket, with a plastic mesh of 3 mm and a total capacity of 0.025 m3, developed by Hexcyl Systems Pty Ltd. (Australia). The baskets were suspended in three cylindrical fiberglass tanks, arranged as shown in the Figure 1, with an open water circuit and aeration with diffusing stones. The tank system was located outside, and the temperature was natural and recorded periodically. Every three days, the bottom and walls of the tanks were cleaned with a broom, to expel faeces and other dirt through the bottom drain. The trial started in early autumn and lasted about 6 months (182 days). (Figure 2)

Figure 2 Mean wet weight (left) and mean test diameter (right) of Paracentrotus lividus fed with the experimental formulated feed and control feed (Ulva spp.). *Samplings where significant differences were found (P=<0.05).
Sampling
Biometric sampling (total wet weight and test diameter) of 30 sea urchins per basket was performed once a month. In December no sampling was performed. Weighing was done with a KERN PRS / PRJ precision and analytical balance. To measure the test- diameter the sea urchins were photographed next to a ruler, and the pixel-centimeter conversion was performed using the ImageJ software. In order to analyze the growth in weight, the Total Wet Weight Gain (TWWG) index and the Total Specific Growth Rate (SGR) index were used:
For the Survival Rate (SR%) the following equation was used:
In the months of September (initial sampling), November, January, February and March, 3 randomly chosen individuals from each basket were weighted, and then, anesthetized by submersion for five minutes in cold water (-1ºC), to proceed with their dissection. With a scalpel, a circular opening was made at the bottom of the sea urchin, and the feces were removed with a slight jet of distilled water. Thus, each individual's gonads were extracted and weighed to determine the gonadosomatic index:
For the analysis of the fatty acid profile, the removed gonads were grouped into pools of 3 units (one pool for each replicate). Thus, for each treatment, 9 individuals were used for this analysis in each sampling moment. These samples were immediately placed in liquid nitrogen for rapid cryopreservation and then stored in a freezer at a temperature of -80 °C.
Fatty acid profile analysis
Fatty acid methyl esters (FAME’s) of total lipids were prepared by acid-catalyzed transesterification using Lepage and Roy method,18 modified by Cohen et.al.19 A (0.3 g) of freeze-dried sample was saponified and transesterified with methanol/ acetyl chloride solution (19:1) and the methyl esters were extracted into n-heptane. The internal standard used was saturated fatty acid heneicosanoic acid (21:0). FAME were separated and quantified using a Varian CP 3800 gas chromatograph (Walnut Creek CA, USA) equipped with an autosampler, a flame ionization detector (250 ºC) and a DB-Wax Polyethylene Glycol Column (Agilent Technologies, S.L., Madrid, Spain) (30 m, 0.25 mm i.d). Injector temperature was maintained constant at 250 ºC, over the 40 min of the analysis. The column was submitted to a temperature gradient, 5 min at 180 °C, followed by an increase of 4 °C/min for 10 min until it was reached 220 °C. Peak identification and quantification was done by using the relative retention times between each FAME from sample tissue and the reference standards for the most common FAMEs (Sigma Kit no. 189-19, ST Louis MO, USA). Fatty acids means are expressed in mg/100 g.
At the end of the experiment 6 individuals/tank were sacrificed and one gonad per individual were collected. The gonad tissue and stored in Formol during 24h and after transferred to ethanol at 70%. The tissue was processed using a standard histologic technique.20 Slides was obtained using a tissue processor (Model Citadel 2000, Thermo Scientific, Nanjing, China) and sectioned (4 µm thick) with a microtome (Model Jung RM 2035, Leica Instruments mb, etzlar, Germany). Slides were stained with haematoxylin and eosin using an automatic slide stainer (Model Shandon Varistain 24-4, Thermo Scientific, Nanjing, China). Mounted slides analysis was performed under a NIKON optical microscope. Eclipse Ci and photographed with a NIKON Ds Fi 2 camera). Mounted slides were scanned with a Hamamatsu NanoZoomer C13140-01 and images were visualized with the NDP.view2 by Hamamatsu Photonics K.K, Shizouka, Japan. The gonad maturity stage was determined following the method described by James et.al.,21 and classified into one of 4 different maturity stages: Stage I: Post spawning; Stage II: Storage cells (nutritive phagocytes) growth; Stage III: Development of reproductive cells; Stage IV: Spawning and pre-spawning.
All results obtained were statistically compared to determine the existence of potential differences between treatments (Student's t-test) when equal variances between treatments, or Mann-Whitney Rank Sum Test, when unequal variances between treatments) with a 95% confidence interval, that is, p <0.05. Fatty acid profile was statistically analyzed using Student's t-test. All analysis were carried out with Sigmaplot® software.
Survival and somatic growth
The survival rate for sea urchins fed with the formulated feed and control (Ulva spp.) was 100%.
Regarding the somatic growth, there was a greater development of sea urchins fed with the formulated feed, compared to those fed with macroalgae (Control). The total wet weight gain (TWWG) of the control was significantly lower than the formulated feed (p<0.05), however no significant differences were found between the total specific growth rate (SGR%) of the sea urchins fed with formulated fish and with the control diet (Table 3).
|
Formulated feed |
Control |
|
|
Total wet weight gain (%) |
56.54 ± 18.06a |
22.87 ± 3.54b |
|
Total specific growth rate (%) |
0.29 ± 0.08 |
0.17 ± 0.03 |
Table 3 Growth parameters of Paracentrotus lividus fed with the experimental formulated feed and control feed (Ulva spp.). (mean ± SD). Letters a, b represents significant difference (c) in the means of sea urchins fed the different diets. Same letter in the line stands for no significative differences (p>0.05)
Gonads development
Since the beginning of the trial, there was a progressive increase in the gonadosomatic index of sea urchins fed inert food. In the case of sea urchins fed with macroalgae, this index decreased during the autumn months, after which it remained stable until the end of the trial (march). In all samples, significantly higher gonadosomatic index was recorded in sea urchins fed with formulated feed (p=<0.05) (Figure 3). Through histological analysis it was found that at the end of the trial the gonads of sea urchins fed the two diets did not show significant differences with respect to their maturation state. All analyzed individuals had gonads in phase IV: Spawning and pre-spawning.
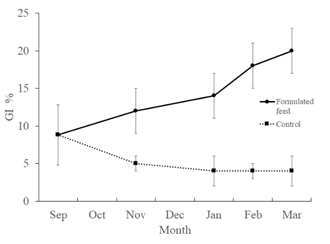
Figure 3 Gonadossomatic index of Paracentrotus lividus fed with the experimental formulated feed and control feed (Ulva spp.).
Figure 4 shows the microscopic appearance of the gonads of male and female animals at developmental stage IV.
Fatty acid profile
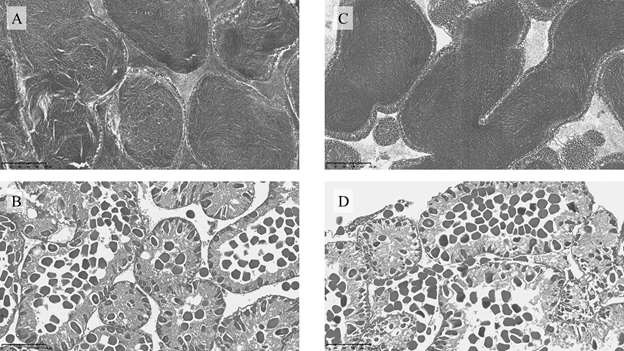
Figure 4 Histological section of gonad from Paracentrotus lividus at the end of the trial, under farm-conditions. A- Control diet, male; B- Control diet, female; C- Formulated feed, male; D- Formulated feed, female.
The profiles of the main fatty acids of the two diets (Formulated fed and Control) and sea urchins gonads fed with the respective two diets are shown in Table 4.
|
|
Diet |
Gonads |
|||
|
Fatty acid |
Formulated feed |
Control |
Formulated feed |
Control |
|
|
14:00 |
2.97 ± 0.10a |
8.6 ± 0.4b |
6.61 ± 1.0a |
6.62 ± 0.25a |
|
|
16:00 |
34.03 ± 0.43a |
19.2 ± 0.3b |
5.33 ± 0.03a |
36.53 ± 3.39b |
|
|
18:00 |
2.38 ± 0.10a |
0.3 ± 0.2b |
2.14 ± 0.10a |
2.21 ± 0.07a |
|
|
41.65 ± 0.45a |
38.0 ± 0.6b |
27.56 ± 2.69a |
26.62 ± 0.04a |
||
|
16:01 |
0.90 ±0.20a |
1.2 ± 0.1b |
4.09 ± 0.07a |
17.98 ± 2.45a |
|
|
18:1 |
18.96 ± 0.04a |
7.7 ± 0.2b |
13.41 ± 0.15a |
58.22 ± 3.42b |
|
|
18:1 |
nd |
nd |
0.02 ± 0.04a |
0.09 ± 0.02a |
|
|
20:01 |
0.91 ± 0.00a |
0.1 ± 0.0b |
8.21 ± 0.48a |
9.14 ± 0.78b |
|
|
21.11 ±0.03a |
16.6 ± 0.2b |
20.15 ± 0.57a |
23.47 ± 0.41b |
||
|
18:2 ω 6 (LIN) |
15.01 ± 0.01a |
9.5 ± 0.2b |
6.98 ± 0.95a |
9.23 ± 2.06a |
|
|
20:4 ω 6 (ARA) |
0.95 ±0.00a |
1.8 ± 0.1b |
0.39 ± 0.03a |
1.38 ± 0.37a |
|
|
16:4 ω 3 |
nd |
nd |
2.51 ± 0.33a |
2.82 ± 0.58a |
|
|
18:3 ω 3 ( ) |
1.20 ± 0.00a |
0.1 ± 0.0b |
0.61 ± 0.03a |
1.08 ± 0.02b |
|
|
18:3 ω 4 |
nd |
nd |
0.61 ± 0.03a |
1.08 ± 0.02b |
|
|
18:4 ω 3 |
0.54 ± 0.00a |
0.2 ± 0.0b |
0.74 ± 0.06a |
2.29 ± 0.04b |
|
|
20:4 ω 3 |
0.29 ± 0.00a |
0.1 ± 0.0 |
0.39 ± 0.03a |
1.38 ± 0.37b |
|
|
20:5 ω 3 (EPA) |
0.36 ± 0.04a |
1.6 ± 0.1b |
0.39 ±0.03a |
1.38 ± 0.37b |
|
|
22:1 ω 9 |
nd |
nd |
2.26 ±0.31a |
2.67 ± 0.45a |
|
|
22:5 ω 3 |
nd |
1.4 ± 0.0 |
0.16 ± 0.14a |
0.58 ±0.05b |
|
|
22:6 ω 3 (DHA) |
11.63 ± 0.15a |
0.2 ± 0.0b |
7.95 ± 1.29a |
0.31 ± 0.16b |
|
|
34.99 ± 0.24a |
26.8 ± 0.3b |
38.13 ± 2.68a |
32.96 ± 0.53b |
||
|
14.24 ± 0.18a |
14.1 ± 0.6a |
13.68 ± 1.66a |
11.83 ± 1.03a |
||
|
20.66 ± 0.05a |
12.1 ± 0.1b |
21.60 ± 0.69a |
17.56 ± 2.29b |
||
|
0.70 ± 0.01a |
1.2 ± 0.0b |
0.63 ± 0.06a |
0.69 ± 0.15a |
||
Table 4 Fatty acid profiles of the two diets Formulated fed and Control and sea urchins (Paracentrotus lividus) gonads fed with the respective two diets, in relative values (%). Mean values. Different lowercase letters show that there are significant differences in the same groups (p < 0.05)
Table 4- Fatty acid profile of the two diets Formulated fed and Control and sea urchins (Paracentrotus lividus) gonads fed with the respective two diets, in relative values (%). Mean values. Different lowercase letters show that there are significant differences in the same groups (p <0.05)
Observing the profiles of the tested feeds the two treatments (Formulated feed and Control) significant differences were found in the sum of MUFA (monounsaturated fatty acids) and PUFA (Polyunsaturated fatty acids) (Student's t-test P<0.05). The formulated feeds has the highest percentage of PUFA fatty acids, with the contribution of 22:6 ω 3 (DHA) and 18:2 ω 6 (LIN) being very relevant to the PUFA content. The Control diet (Ulva spp.) has values of 20:5 ω 3 (EPA) significantly higher than inert foods (Student's t-test, P<0.05). Regarding the class of fatty acids SFA and MUFA, the main highlights are the high content of 16:0, 18:1 ( ) in the two analyzed diets.
The first highlighted result is a high content of polyunsaturated fatty acids (PUFAs) in the gonads of the sea urchins fed of both diets tested. In the case of the formulated feed, the greatest contribution to the high content of PUFAs is made by the 22:6 ω 3 (DHA), which is significantly lower (T-Test, P<0.05) in sea urchins fed with macroalgae. In the sea-urchins fed with Ulva spp., the PUFA content is mainly due to 18:2 ω 6 (linoleic acid) and 16:4 ω 3, which is statistically superior to animals fed with formulated diet (Student's t-test, P<0.05). The value of 20:5 ω 3 (EPA) is much lower than the DHA for both treatments.
Regarding the SFA fatty acid profile, no significant differences were found between the gonads of sea-urchins fed the two diets (Student's t-test, P<0.05), with the 16:0 fatty acid being the most abundant in both treatments.
Survival and somatic growth
Individual size, eating behavior, physical environment, food availability, and food quality all play a role in sea urchin somatic growth. Sea urchins have a slow growth during the late juvenile and adult phase so, developing an adequate food for them is critical to the aquaculture.22 Providing the nutrients essential for sea urchin somatic growth in a stable inert fed is the first step when producing an optimum diet. Since differences in somatic growth have been attributed to differences in food quality, studying the responses of sea urchins to specific nutrients in prepared meals to estimate the relevance of feed components for growth potential is needed.23,24
In this work, one formulated feed for the cultivation of Paracentrotus lividus was developed in order achieve good results of somatic growth during the ongrowing culture phase, therefore, the development of optimized gonads for human consumption was not the primary objective.
Survival of sea urchins in this trial was 100% for all treatments. Other studies analyzed also obtained good results in terms of survival, thus showing the resistance of these organisms at this stage of life in different cultivation conditions.2,7,25
Direct observations showed a good acceptance of sea urchins in relation to the tested inert fed and the control (Ulva spp.). The two inert diets have a high content of ingredients that are naturally found in the diet of wild sea urchins, such as the macroalgae Ascophyllum nodosum and Ulva spp. (this one collected in the tanks of the IPMA facilities), which increase the palatability of inert food. Cyrus et al.,26 also included 20% of Ulva sp. in their tested formulated fed, verifying the increase in acceptability by sea urchins.
Regarding the somatic growth, it was found that sea urchins fed with inert food showed greater growth compared to those fed with Ulva spp. The better performance of inert food be due to the higher protein content compared to macroalgae ( ; ). The importance of protein content in somatic growth was also observed by Schlosser et.al. and Cyrus et.al.5,12
Hammer et.al.27,28 found that a protein content lower than 21% DM (% of total dry matter) compromised the growth and survival of our sea urchin (Lytechinus variegatus), with optimal results with observed diets with protein of 31% DM, very close to the inert fed formulated in this work. The protein available in the formulated feed comes mainly from wheat and corn gluten vegetable protein, these ingredients totaling 24.5% of the composition of the formulated fed. Gluten is a high-protein ingredient with an interesting amino-acid profile. It contains great amount of sulfur amino acids and glutamine, which has been shown to boost gut health and immune modulation.29 Due to its protein quality, low price, and the need to minimize the use of animal protein, gluten is a widely used ingredient in the formulation of inert food in aquaculture.30–32 Some similar studies with inert feds for sea urchins tested the use of other protein sources rather than exclusively vegetable. Fernandez and Boudouresque13 analyzed several parameters of development of P. lividus when fed with food of animal, vegetable, and also mixed origin, having observed that the greatest somatic growth occurs with ingredients of animal origin. The sea urchin P. lividus is primarily herbivorous in the wild, but it can augment its diet with animal-derived foods such as mussels, polychaetes bryozoans and other macroalgae epiphytes, small crustaceans, and even opportunistic feeding on dead vertebrates, thus increasing the protein uptake.22 By incorporating vegetable protein such as maize and wheat gluten, it was possible to increase the protein content of the formulated fed to 32.62% of total dry matter, optimal levels for somatic growth.27,28
The higher level of energy provided by the inert food could also be relevant in the somatic growth of sea urchins. Heflin et.al.,25 suggests that variations in dietary nutrients and energy differentially affect organismal growth and growth of body components, having observed that higher energy diets produced lower FCR (Food conversion ratio), that is, food with more energy allows a better conversion of food into somatic growth.25,33
The sea urchins’ gonads are responsible for both the creation of gametes and the storage of nutrients within nutritive phagocytes, which are gonadal cells. The nutrients held within the nutritive phagocytes are needed to produce gametes for reproduction as sea urchins start gametogenesis, consequently the nutritive phagocytes reduce in size.22,34 When the gonads are filled by large nutritive phagocytes, before gametogenesis has fully progressed, the quality of the gonads is considered optimal for human consumption.22,35,36
In the present work, the evolution of the gonadosomatic index was recorded monthly between September and March, which corresponds to the period from the beginning of autumn to the beginning of spring. It is at this time of year that the recovery of the gonads usually occurs after the end of the spawning period.37,38 On the south coast of Portugal, the minimum of the gonadosomatic index occurs in January, when more than 75% of the population is in the recovery phase. Gonad maturation occurred throughout winter–spring, followed by a single but prolonged spawning season during spring–summer.39
In this study, a pattern of variation in the gonadosomatic index like other consulted studies was observed for sea urchins fed inert food.39,40 However, an increase in this index was observed since the month of September, which differs from that observed in the natural environment. According to Machado et.al.,39 the increase in GI only occurs in November, that is, 2 months later. This could be due to the difference in the water temperature pattern. In this author's work, the average water temperature increased until October, with a decrease only in November. Under the experimental conditions of this work, the temperature started to decrease as early as September, with its monthly average dropping from 22.7ºC to 18.2ºC. Although the mechanism is not yet fully understood, it is thought that the gametogenic cycle is influenced by a set of abiotic factors, with temperature being one of these factors. According to several authors, gonadosomatic indices and maturation decrease with lower sea temperature and photoperiod.38,41
The germinal epithelium of the gonads is composed by germinal and also somatic cells called nutritive phagocytes, which are responsible for structural and nutritional changes in the germ cells throughout the cell in gametogenesis.36 A biochemical structure of the gonads undergoes alterations with the temperature mechanism due to its strategic nutrient storage. In females, changes occur mainly in the nutritive phagocyte biochemical composition. In males there is a change in the number of gametes available in the germinal epithelium.42
Despite the dependence of gametogenesis on abiotic conditions, the nutritional quality of the food may have an influence on its regulatory mechanism. In the present work, significant differences were observed in the evolution of the gonadosomatic index of sea urchins during the study period. This index evolved markedly in sea urchins fed inert food. In animals fed exclusively with seaweed, the development of the gonads was very reduced. Bearing in mind that the abiotic conditions were the same for both treatments, the responsibility for the differences in the gonadosomatic indices should be the biochemical differences in the food provided. Proximal analysis revealed that the inert feed had higher levels of protein and lipids than the natural food (Control-Ulva spp.). With a more nutritionally rich food, nutrients can be used both in terms of nutritive phagocytes and in the production of gametes, a favorable situation for greater development of the gonads. Raposo et.al.43 also found a close relationship between food and the gonads development. In this author's work, it was found that sea urchins fed with a maize and spinach-based diet, richer in lipids, had gonadosomatic indices significantly higher than natural (macroalgae) or semi-natural (macroalgae and vegetables) diets, with a lower lipid content. Mendes et al.,8 also verified that the inclusion of maize in the diet of sea urchins provides higher somatic gonad indices when compared with a diet based only on Ulva spp.
Despite boosting the gonadosomatic index, feeding inert food has little effect on gamete maturation. Araujo et al. (to be published) studied gonad development in sea urchins under different environmental contexts and analyzed the role of environmental variables in gonad maturation. However, changes in gonad maturation were not caused by the fed offered (Ulva spp and the same inert food utilized in the same trial). These findings are also consistent with those of Cyrus et al.26 and Shpigel et al.,44 who examined the histology of T. gratilla gonads that had been given fresh Ulva and inert diets containing Ulva, respectively.
Fatty acids (FA) are essential molecules that play an important role in immunological and physiological functions. Microalgae and marine algae are an important source of FA in marine food chains and, directly or indirectly, in human nutrition, especially due to their high PUFA content.45 This class of FA are of great importance in the prevention of cardiovascular diseases, and are also important antihypertensive, anticancer, antioxidant, antidepression, antiaging, and antiarthritis effects.45–47 Sea urchins are marine organisms already known as a source of PUFAs, constituting a highly nutritious food for humans. For sea urchins this class of FA plays an essential role in the growth and development of the gonads, being able to perform biosynthesis of some of these molecules.48 In this work, the high content of PUFAs in the gonads of sea urchins was confirmed, with linoleic acid (18:2 ω 6) being the most abundant FA, regardless of the food supplied. Candeias-Mendes et al.8 also found high levels of linoleic acid (18:2 ω 6) in the gonads of sea urchins fed with maize and Ulva spp., these values being much higher than the percentage obtained in this work. In Anedda et.al.49 the values of this fatty acid were lower, not reaching 5% of total fatty acids.
The second most common PUFA fatty acid in the gonads and sea urchin was docosahexaenoic acid (22:6 ω 3), commonly referred to as DHA. This fatty acid is present mainly in marine organisms, due to the production by the photosynthetic organisms, which, through the marine food chain, will supply this fatty acid to the heterotrophic organisms, the biosynthesis by the latter being insufficient for the metabolic needs.50–52 DHA is considered an extremely important fatty acid for human nutrition, being essential for the proper development and maintenance of brain functions. A diet low in DHA contributes to the decline of cognitive abilities with age and to the onset of several diseases such as attention deficit hyperactivity disorder, cystic fibrosis, phenylketonuria, unipolar depression, aggressive hostility, and adrenoleukodystrophy.53 For marine animals, this fatty acid, like others in the PUFA family, is essential for reproductive function, and a deficient nutrition in these fatty acids will affect the broostock fecundity, fertilization, hatching rate and viability of the eggs, as well as growth and development of larvae of marine organisms.54,55 In this work, relatively high values of DHA were observed in the gonads of sea urchins fed with formulated fed. For those fed with macroalgae, the values were very low. These results clearly reflect the importance of diet as a source of DHA. In Raposo et.al.43 DHA values in the gonads of sea urchins were much lower than those recorded in the present work, reflecting well the FA profile of the food tested. In any of the diets tested by Raposo et.al.43 the DHA value was null.
According to Kabeya et.al.,48 the content of DHA in sea urchins is relatively lower than that of EPA and ARA, because sea urchins’ fatty acid desaturases (key enzymes in the biosynthesis of long chain-PUFA) activity is a typically Δ5 and Δ8 pathway. DHA biosynthesis is carried out through the percursor 22:5 ω 3, following the Δ4 pathway; however this type of reaction was not identified in this species.
The inclusion of Schizochytrium biomass in the formulated fed was crucial for the supply of DHA in the sea urchin diet and, consequently, for its incorporation into the constitution of the gonads. Differential supply of DHA in both treatments resulted in marked differences in gonad growth. A diet composed exclusively of Ulva spp. it will therefore not be suitable for good development of the gonads. Candeias-Mendes8 also found low gonadosomatic indices in sea urchins fed on Ulva spp. In the same work, a diet composed of a mixture of the same macroalgae with corn was also tested, however the DHA content in the gonads remained at very low levels, not allowing to reach the gonadosomatic indices achieved in the present work.
EPA fatty acid is considered, like DHA, also an ω3 fatty acid of great importance for human health, contributing to an adequate fetal development, preventing cardiovascular diseases, maintaining good brain function, and reducing inflammatory disorders.56 For sea urchins, EPA (like DHA) plays an essential role in larval survival and development.48 In this work, a relatively low content of this fatty acid was verified in the gonads of sea urchins, mainly in animals fed with formulated food. Higher values were reported in Raposo et.al.43 and Candeias-Mendes.7 Sea urchins can biosynthesize EPA through the desaturase action FadsA, having as raw material the precursor 20:4 ω 3,48 however the presence of this fatty acid in the food was greatly reduced, mainly in formulated foods, thus explaining the relatively low content in the analyzed gonads. The ratio of DHA:EPA content is also a very important parameter in nutritional terms. In fish, it is already known that an unbalanced DHA:EPA ratio can destabilize the general metabolism of lipids, promoting the development of malformations and generalized pathologies.57 Also, an imbalance in the structural composition of the phospholipids caused by a higher quantity of EPA in comparison to DHA could compromise the larvae's normal growth and quality.54 For human health foods with different DHA/EPA ratio can be used depending on the nutritional strategy. A diet with DHA:EPA in the ratios of 1:1 and 2:1 reduced inflammation and oxidative stress primarily at the 1:2 ratio. Antioxidant enzyme activity increased in erythrocytes, abdominal fat and kidneys, with varying degrees of increase depending on the EPA:DHA ratio.58 In the present work, a great difference was observed between the DHA:EPA ratios observed in the gonads of sea urchins fed with formulated feed and natural food (control-macroalgae). In the formulated fed, the value of this ratio was approximately 20:1, for the control this value was 0.23:1. In Candeias-Mendes et.al.8 and Raposo et.al.43 the DHA: EPA ratios were very similar to the results obtained with the control diet of the present work. These results are easily explained due to the addition of DHA in the formulated feed tested, incorporated through Schizochytrium microalgae biomass. This feed constituent may have been the main responsible for the excellent development of the gonads.59
In conclusion, the formulated diet demonstrated great promise in terms of growth and gonad development. The addition of polyunsaturated fatty acids (PUFA) to feed appears to be beneficial to the development of the gonads, resulting in a significant increase in the gonadosomatic index. Future trials will be undertaken with the aim of improving inert feed for sea urchins, namely the formulation of a feed with nutritional characteristics that promote faster shell growth rather than promoting gonad growth. This formulation may involve increasing the percentage of the mineral fraction in the rations.
This study was funded by FUNDOAZUL (OURIÇAQUA, FA__05_2017_009).
The authors have no competing interests to declare that are relevant to the content of this article.

©2023 Araújo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.