Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 4
Department of Aquaticecology and Environnent, University Hassan II, Morocco
Correspondence: Sanaa Bhaby, Department of Aquaticecology and Environnent, University Hassan II, Casablanca, Morocco
Received: June 18, 2015 | Published: August 6, 2015
Citation: Bhaby S (2015) Synchronous Reproduction in Mussel (Mytilus galloprovincialis) Population from Atlantic Ocean of Morocco. J Aquac Mar Biol 2(4): 00031. DOI: 10.15406/jamb.2015.02.00031
We investigated the gametogenesis of a natural population of Mytilusgalloprovincialis in the Atlantic central zone (Cape Beddouza). We found that the cycle of reproduction was characterized by a synchronous gametogenesis. From March 2009 to March 2011, we histologically analyzed the status of gonad development and the reserves cells at the peak of spawning. To determine the status of gonad development, we used three biological indecies parameters: the gonadic index, the condition index and the ADG index. The gonadic index was the highest in winter. The condition index was the lowest in spring. The ADG index was the highest in autumn. We found synchrony of these indices in majority of sampled M. galloprovincialis. The found synchrony was unique in the populations other waters in Moroccan coast, where gonad development cycle was individually different. The synchrony of reproductive cycle in the population maybe a result of the consistent seasonal pattern of environmental variable along the cape.
Keywords: Mytilusgalloprovincialis, Reproduction, Condition index, Gonadic index, ADG cells, Histology Atlantic Ocean
In Moroccan water, M. galloprovincialis distributed from the Mediterranean Sea to the Atlantic Coast.1 There are both natural population and aquacultural stocks of M. galloprovincialis in this region. In 2005, the gradually increased production of M. galloprovincialis reached 34 tons in Moroccan water, but it was at stand still until 2009.2 In 2010, the production decreased to less than half of the maximum in recent years.2 To maintain a fragile balance between captures and natural renewal of stocks, available information in the M. galloprovincialis in Moroccan water is not sufficient yet.
The natural population of M. galloprovincialis has been under potential pressure of local consumption, commercial fisheries and coastal development. However, it has not been well studied in this area. Until 2000, there was only one scientific publication about the general reproductive biology of the M. galloprovincialis, discussing the multiple peaks of gonad maturation.3 After 2000’s, there were two papers of reproductive biology of M. galloprovincialis in coastal water of Mediterranean Sea and the Atlantic coast of southern Morocco with detail investigation with histological analyses.4,5 There are still many observed habitats and aggregations of M. galloprovincialis along Moroccan coast, and those few investigations are not sufficient to understand the natural populations and to monitor their status.
The reproduction of fishery targeted mussels, including M. galloprovincialis has been studied in European Atlantic waters .6 On the other hand, not many scientific investigations were conducted on the reproductive cycle of mussels in African Atlantic waters. About Mytilus spp. the seasonality and duration of the reproduction were explained by effect of the spatiotemporal variation of the ambient environmental conditions in their habitats. The temperature and the food were suggested as the major exogenous factors which control and regulate the duration and the synchronization of reproductive cycle of the mussel.7-9
The histology is necessary for the better investigation in development of gametes, and the quantitative estimations are important because they eliminate the subjectivity and the problems associated to the description. The study of the condition index is one of the methods retained to evaluate the potentialities of weight growth bound to the reproductive cycle. The reproductive tissue of sexually matured M. Galloprovincialis accounts for more than 59% of the weight of the soft tissue.10 or more than 95% of the mantle. Reserve cells in the mantle, the ADG cells, has been observed in histological specimen, and the seasonal variation of number of ADG cells has been investigated .4,11 The stored nutrients are released during gametogenesis; this mobilization of reserves is followed by a phase of active reserve during the period of sexual rest. The process is also able to be observed through histological analyses. Unfortunately, investigation in the mussels with histological analyses is limited in Moroccan water excepting in Mediterranean Sea population of M. galloprovincialis .4 No work has been done in the Atlantic region in Morroccan water. This study investigates the annual successive cycles of the reproductive activity of gametes in the Atlantic coast of Morocco. The status of gonad development and the reserves cells at the peak of spawning were histologically analyzed.
Study area and sampling site
The coastal shelf of Cap Beddouza (32° 32’ 24" N; 9° 16’ 48" W), also called Cape Cantin, was our study area. This cape is isolated from major human activities and away from any industrial or agricultural activities because of the landscape. The coast line is steep, surrounded by high cliffs reaching 60 m. The area is the part of the Middle Atlas mountainous area, extended into the Atlantic Ocean. Despite of the steep coastline, sea bed slope are from 0.3 to 0.7%. The costal shelf broadly extends to 30 km of the coastline, and 100-m isobath is located at 20 km of the coast. The major current comes from the northwest. The semi diurnal tide has a maximum level (3.60 m) in spring tide and 0.7 m in neap tide. The prevailing winds are generally from the north and moderate. In winter, wind direction changes to South-West wind, and the most intensive wind prevail from the southwest.
Samples were collected made every month along the coast of Cap Beddouza from March 2009 to March 2011 (Figure 1). Every sampled set at individual survey contained thirty adult individuals of M. galloprovincialis. The sampling was made in low tide, and samples were transported in the laboratory for measurement, dissecting and histological analysis. During sampling, temperature was recorded at each survey at 30 cm of depth of the sampling site. It was measure during conductometer (WTW LF197).
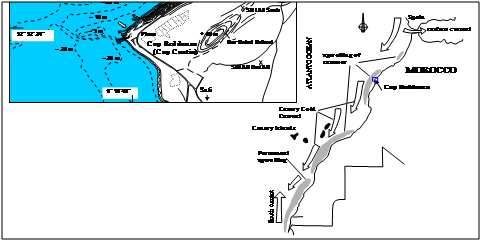
Figure 1 Situation plan of the sampling place Cap Beddouza; the steep coast lined with big cliffs of 60 m.
In laboratory, the weight was measured before the opening of the shell. After opening mussel, the adductor muscle was removed from shell (Wc, g) to weigh fresh flesh (Wf, g) and shell (Wt, g) separately. We applied condition index to quantify the development of gonasomatic tissue relative to the content within the individual M. galloprovincialis :
The individual M. galloprovincialis was microscopically examined for gonadal development. The gonadal tissue for every M. galloprovincialis was fixed Davidson’s solution during 24 hours. After dehydration in a graded ethanol series before embedded in paraffin, the tissues were sliced in 2 µm thick and then stained by using hematoxylin andeosin. The sex of the specimen was determined during the histological processing. To determine the reproductive states of M. galloprovincialis, gonad development stage was classified into seven stages using the method defined by.11 and applied in Ojea et al.12 Benomar et al.13 and Bhaby et al.4 Gonadic index (GI, Seed, 1975) was applied to each stage of maturity: Index 1 (stage 0 and stage IIID); Index 2 (stages I, II, IIIB and stage IIIC), Index 3 (stage IIIA).GI were calculated as the following formula of Seed (1975):
Where ni: Number of individuals at each stage of reproductive cycle, Si: score of the stage and N: total number of individuals. Monthly mean of the GI were calculated, and the time series of GI was analyzed through the seasons. Gametogenic development was indicated by increases of the monthly GI while decline in the time series indicates that occurrence of spawning. To quantify adipose-granular ADG state of M. galloprovincialis, we used the method suggested by Bignell et al.14 Four levels of ADG states were determined: Index 4 (ADG abundant cells constitute the major part of the connective tissue) index 3 (ADG common cells) index 2 (ADG cells spread over the mantle) index 1 (ADG trace cells) index 0 (ADG imperceptible cells).
In all statistical analyses, R version 2.15.2 (R. Core Team) was applied. After exploratory analyses and normality test with adequate transformations, seasonal variability of the condition index, were tested by one-way ANOVA. The Kruskal-Wallis test was applied to examine seasonal differences in gonadic and ADG indices with consideration in the normality of the data. The Chi-square test was used to compare the observed variation of the sex ratio to the proportion 1:1. All hypotheses were tested at the level of α= 0.05.
Temperature
The water temperature changed between 15°C and 21°C from March 2009 to February 2011 (Figure 2). The temperature was lower than 17°C in winter and spring and higher than 17°C in summer and autumn. The maximum was in September 2009 (21°C). On the other hand, the minimum temperature was recorded in March 2009. There was a distinct difference in the spring to summer temperature change between 2009 and 2010; the temperature from May to August was highly variable between 17°C and 19°C in 2009 though it raised as the change of the season in 2010.
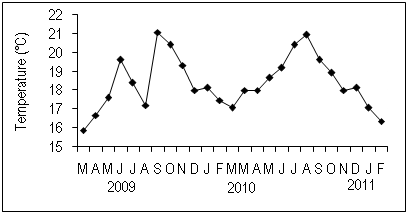
Figure 2Variation of the temperature of the surface sea water in Cap Beddouza between March 2009 and March 2011.
Sex ratio
In 750 specimens observed by microscope, 345 females, 400 males and 2hermaphrodites were observed with three unidentified individuals. The hermaphrodites are recorded as females. The sex ratio was not significantly different from the theoretical sex ratio, 1/1 (Table 1).
|
Independent Variable |
Sex |
Observed Staff |
Theoretical Number |
ddl |
Χ2 |
P-Value |
|
Cap Beddouza |
Female |
347 |
373.5 |
1 |
1.88 |
0.052 |
|
Male |
400 |
373.5 |
Table 1 Chi-square test on the sex ratio of sampling mussels in the study site Cap Beddouza
Reproductive cycle
We observed all gametogenic stages in the specimen.
The gonadic activity shows the effective presence of all stages but with a very marked difference in times (Figure 3). The stage IIID and stage 0, post-spawning stages were observed from spring to summer. These post-spawning stages were most frequently observed in early summer, from May to July. The stages I and II were highly observed in last summer and in autumn. In last autumn and in early winter, November December and January, stage IIIA took place up to a maximum. The spawning (stage IIIB) was continued with the starting up of the gametogenesis (stage IIIC) at the beginning of the winter throughout of the spring. The highest proportion of mussels sampled in stage IIIB or stage IIIC was respectively observed in February, March and April.

Figure 3 Monthly distribution of proportion of Mytilusgalloprovincialis between March 2009 and March 2011.
Gonadic index
The gonad index illustrated in the Figure 4, was showed high value (2.6) in winter which the histology indicated an advanced level of maturity (stage IIIA). The lowest value (1.3) of gonadic index was recorded in summer, which revealed the period of post-spawning (Figure 5). The Kruskal-Wallis test indicated that there were, in relation to the seasons, there was a significant contrast of gonad activity (p-value<0.05). This result was also well illustrated by the box plots (medians particularly differentiated).
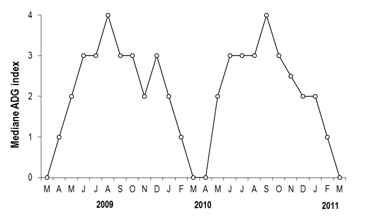
Figure 4 Monthly evolution of the ADG index of Mytilusgalloprovincialis between March 2009 and March 2011 in Cap Beddouza.
Adipogranular index (ADG)
The Adipogranular cells (Figure 4) were imperceptible in spring from February to April. They began to manifest so scattered (index 2) in last spring in May, and in November and January. Their presence becomes dominant (index 3 and 4) from June to October (years 2009 and 2010) (Figure 5), the peak being reached in August and September, which was the consequence of the invasion of cells reserves in the mantles. The proliferation of those cells was indicated the end of a cycle and announced at the same time a start of new cycle. However, to perform a seasonal approach, we have been prepared the corresponding box plots; which they showed a marked asymmetry in the distribution of the ADG index. The Kruskal-Wallis test was validated the observed seasonal box plots differences (p-value<0.05).
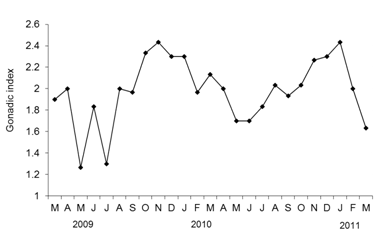
Figure 5 Monthly gonadic index of Mytilusgalloprovincialis in Cap Beddouza between April 2009 and March 2011
Condition index
The condition index (Figure 6) oscillated regularly and gradually around the average of 44%. The maximum value was recorded in winter and in autumn, it was produced in January 2010, where are corresponded to the gonadal maturity in almost all individuals. The accumulation of reserve cells which was observed in September 2010 was also demonstrated by a maximum value of condition index. In the spring, the condition index measured was lower. This last period corresponded to the phase of massive spawning. It was recorded in April and May. Condition index showed significant responses to the season which was perfectly illustrated by the box plots (ANOVA, p-value<0.05) (Figure 7).
Cap Beddouza divided up in a characteristic environment of the Moroccan Atlantic center coast, where characterized by upwelling in summer. This work is the first studies have been carried out the biology of Mytilusgalloprovincialis and especially its reproductive cycle in this area. To know all details of the reproductive cycle is important to make a success of the breeding. It can be used to master effective techniques for the conditioning; induction of spawning and embryonic development. We analyzed the gametogenesis processing of mussels in relation to their reserve accumulation and weight differences. Seasonal variability of these analyses was also evaluated. This work showed that in the center of Morocco (Cap Beddouza), the mussels were characterized by a synchronous gametogenesis broadly overlapping reproductive cycle, with gametogenesis taking place over the entire year. The found synchrony was unique in the populations other waters in Moroccan coast, where gonad development cycle was individually different.4,15
To determine the status of gonad development, we used three biological indecies parameters: the gonadic index, the condition index and the ADG index. On the basis of results, we can say that the reproductive cycle of the natural population M. galloprovincialis in this area is particularly sensitive to the event of the seasons. The gametogenesis begins in summer, continues until the end of autumn. This event is coincided with the higher presence of ADG cells and the beginning of the increase of condition index. The mussels Mytilus galloprovincialis contained ripe gonads at the beginning of the winter until spring when the peak of spawning was observed. In fact, the spawning start in winter and remains till the end of the spring. This report is in accordance, in a similar result, with the observations of Villalba A.6 Seed R.16 Shafee MS .17,18 In particular, the fold of the ADG cells and it’s collapse of the condition index coincide with mass spawning period; this correlation was already identified by Bignell et al.14 in their work from English coast and Bhaby et al.4 in south of Morocco. The massive spawning is translated by a notable hollow of the condition index. Besides, from May to July, the gametogenesis is enlivened and shaken: all stages are present and there is ascendancy, from the final phase to the phase of initiation. We can reasonably connect this continuity with the oceanographic regime which reigns in this region, seen that the temperature undergoes sensitive variations during this period. It joins the conclusions of the following authors: Lemaire et al.9
The outcome of this work owes to the entire staff of the l’Institut national de la Recherche Halieutique Morocco. That each finds here the expression of our profound gratitude.
None.

©2015 Bhaby. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.