Journal of
eISSN: 2378-3184


Research Article Volume 3 Issue 2
Department of Business and Primary Industries, Melbourne Polytechnic, Australia
Correspondence: Sadiqul Awal, Bachelor of Agriculture & Technology degree program (Aquaculture), Department of Business and Primary Industries, Melbourne Polytechnic, Cnr Cooper St & Dalton Rd, Epping, Victoria 3076, Australia, Tel 61392691162
Received: October 27, 2015 | Published: November 20, 2015
Citation: Awal S, Christie A (2015) Suitability of Inland Saline Ground Water for the Growth of Marine Microalgae for Industrial Purposes. J Aquac Mar Biol 3(2): 00063. DOI: 10.15406/jamb.2015.03.00063
Australia enjoys large inland areas rich in saline waters with potentials to marine aquaculture. In this study, these potentials were examined. Initially 9 different species of marine micro algae from three different divisions, namely Chlorophyta, Bascillariophyta and Chrysophyta, were cultivated using batch culture techniques to examine their growth performance in inland saline ground water (ISGW) with f/2 algae culture media. Dunaliealla tertiolecta was found to have highly significant growth performance while Nitzschia closterium showed significantly negative growth. In general, the average cell densities of all species of Chlorophyta were found to be higher compared to all species of Chrysophyta and Bascillariophyta. Significant differences of lipid were found to be in the Chlorophyta group compare to other two groups. The study successfully demonstrated that the marine microalgal group of Chlorophytes can be successfully grown in ISGW. However microalgae cultivation in ISGW still has enormous potential despite the negative growth of two diatom species and comparatively slow growth of Chrysophytes algae in this study. More controlled research on different groups of microalgae would be a good investment and a synergistic approach is recommended, including water chemistry, hydro-geology, soil-water interactions of and nutritional profiles of microalgae in ISGW.
Keywords: Microalgae, Saline ground waters, Chlorophyta, Chrysophyta, Bascillariophyta, Diluted coastal water, Nitzschia, Marine microalgae, P. tricornutum, Industrial purposes, C. fusiformis, ISGW, Isochrysis, Ground water, Australia, closterium, D. tertiolecta,Chlorophytes
ISGW, Inland Saline Ground Water; ASP, Aquatic Species Program; NDSP, National Dryland Salinity Program; ISO, Isochrysis; HCO3-, Bicarbonate; SO4++, Sulphate; Si, Silicon; K+, Potassium
Marine microalgae play a vital role in the ecology of the planet, accounting for approximately 45% of global primary productivity.1 They are an important food source and feed additive in the commercial rearing of many aquatic animals, especially the larvae and spat of bivalve molluscs, penaeid prawn larvae and as an enrichment diet for live food organisms such as rotifers.2-5 The importance of microalgae to aquaculture is not surprising, since algae are the natural food source for these animals. Although several alternatives for algae exist (such as yeasts and microencapsulated feeds), live algae are still recognized as being one of the best food sources and/or enrichment diets available.1,3,6 Microalgae have also been seen as a valuable foodstuff for terrestrial animals since the early 1970s, since some microalgal properties allow superior usage of available nutritional content, thus improving overall health.7,8 In addition to its usages in aquaculture, the use of microalgae as a source of renewable energy is certainly not a new concept; during the 1980s, the U.S. Department of Energy initiated research through the Biofuels Program: Aquatic Species Program (ASP) on the use of microalgae as a source of fuel. The ASP collection contained over 3,000 strains of microalgae at its peak, of which 300 species of microalgae were short-listed and earmarked as suitable for experimentation for the production of biodiesel .9
The importance of microalgae in biofuel production is now well established.10,11 Australia is blessed with vast marine water resources, though lacks suitable inland freshwater sources.12 Unfortunately, much of Australia’s arable lands are subject to secondary salinisation from the agricultural practices of land clearing, irrigation and the replacement of deep-rooted native perennial vegetation with shallow-rooted annual crops.12 As stated by Cordover 13 mobilization of geologically stored salt and rising water tables in the Murray Darling Basin have resulted in the production of highly saline groundwater’s that are beyond the tolerance levels of most non-marine plants and soil-based animals, reducing the possibility of conducting sustainable or viable farming in those areas. The damage and costs of rising water tables and soil salinisation were summarized in the Salinity Audit of the Murray Darling Basin.14 Much of the mobilized salt remains in the landscape or is diverted into irrigation areas, where the total economic impact was estimated at $46 million a year and is expected to rise over the next century. Lands salinised and waterlogged by both irrigation salinity and dry land salinity in the Murray Darling Basin and Western Australian Wheat belt comprise hundreds of thousands of hectares that no longer support traditional crops. In some cases the salinity is too severe to support any terrestrial plants that can be used for crops, pasture or wood and limits the potential for any return of the original ecological biodiversity. Much of this land is considered to be irretrievable for farming.
The enormous problem with rising saline groundwater has achieved increased national recognition in Australia.15
and resulted in the establishment of Australia’s National Dry land Salinity Program (NDSP) in 1993, which was formulated ostensibly to improve research, development, extension and coordination to better manage dryland salinity across Australia. This in turn led to the establishment of a unit known as OPUS (Options for the Productive Use of Salinity).16 in which the potential for the development of inland aquaculture was formally recognized. Therefore, saline groundwater and evaporation basins can be considered together as a resource as well as an environmental expense. Use of these inland marine waters for aquaculture, especially those waters with an ionic composition close to that of seawater, have been considered as a potential use of saline evaporation basins for nearly a decade.13 In response to this issue, research has been undertaken into the use of inland saline groundwater for the aquacultural production of finfish, crustaceans, molluscs and seaweed .13,17-24 but significantly little information has been produced to date on the production of marine microalgae in inland saline groundwater. With this in mind, the aim of this study is to examine the suitability of inland saline groundwater (ISGW) for the production of a range of marine microalgal species that have been drawn from a broad variety of divisions and to prove the hypothesis that some groups of marine microalgae are particularly suited to being grown in ISGW.
Microalgae from three algal divisions were used for this experiment. The experiments were conducted at Melbourne Polytechnic’s (formerly known as Northern Melbourne Institute of TAFE, NMIT) Live Production Laboratories from August to October 2013, using inland saline ground water (ISGW) which was taken from a Victorian groundwater monitoring bore (No. 116677), located in Koonda, 3669 (145°45′02″E, 36°28′30″S). The ionic concentrations of magnesium, sodium, potassium and calcium were determined by atomic absorption spectrometry (Varian AA20, Melbourne). Chloride was determined via volumetric titration using a standard silver nitrate solution with dichlorofluorescein as provided by Vogel.25 with the remaining ions determined by the use of a Merck Spectroquant NOVA 60 spectrophotometer (Merck KGaA, Darmstadt, Germany), with tests conducted using a number of different test kits as appropriate, including Sulphate (method no. 14564), Phosphate (method no. 14543), Fluoride (method no. 14557), Ammonia (method no. 14752), Nitrite (method no. 14776) and Nitrate (method no. 14773).
Pure cultures of nine microalgal species (Dunaliella tertiolecta, Tetraselmis suecica, Nannochloris atomus, Nannochloropsis oculata, Isochrysis galbana (T.iso), Pavlova lutheri, Phaeodactylum tricornutum, Nitzschia closterium and Cylindrotheca fusiformis) were purchased from the Australian National Algae Culture Collection of the CSIRO (Commonwealth Scientific and Industrial Research Organisation) and grown in batch cultures at 20 ± 2°C, under light provided by cool white fluorescent tubes (Philips LifeMax TLD 36W/840, North Ryde, New South Wales) at an intensity of between 4000-4500 lux, as determined by a light meter (Model no: 05132201, Dick Smith Electronics, Sydney, New South Wales) with a 12D:12L photoperiod and aerated with non-heated atmospheric air from a side-channel blower Esam S.P.A, Uni Jet 75 CE, Esam Australia Pty Ltd, Thornbury, Victoria). Salinity was maintained at 19.6 ppt using sterilized freshwater. The collected ISGW was not sterilized, though culture mediums, vessels and all equipment used directly to culture the microalgae were sterilized by autoclave (Tangent Tiger, Atherton, Thornbury, Victoria) at 121°C and held at 125 k Pa of pressure for 20 minutes.
The water was enriched with f/2 medium.26 from Alga Boost 1000 × f/2 concentrate, (AusAqua Pty Ltd, Wallaroo, South Australia) to manufacturer’s specifications (2mL/L culture medium) and, in the case of the diatoms, the culture water was supplemented with Trace Nutrient/Silicate Concentrate, formulated by Florida Aqua Farms, Inc .27 For each species, control treatments were also established which consisted of ISGW at the same salinity level (19.6 ppt) without any additions of the f/2 nutrient. Algae used in the experiments were grown in 500 ml Erlenmeyer flasks. Immediately after inoculation, an initial count of cell density (cells. ml-1) was taken with a Neubauer haemocytometer and culturing of the microalgae was then commenced, with the first counts of cell density and growth rate being carried out two days after inoculation and every alternate day thereafter.
All treatments were run using a completely randomized design, with three replicates and one control for each species being tested. For simplicity, we divided treatments up into various segments based on the divisions of algae that were being tested. Treatment 1 consisted of three algae species from the division Chlorophyta (green algae); Dunaliella tertiolecta, Tetraselmis suecica and Nannochloris atomus; Treatment 2 consisted of three algae species from the division Chrysophyta (golden algae); Nannochloropsis oculata, Isochrysis sp (ISO) and Pavlova lutheri and Treatment 3 consisted of three species of Bacillariophyta (diatoms); Phaeodactylum tricornutum, Nitzschia closterium and Cylindrotheca fusiformis. Cellular density was determined by counting three aliquots of cultures using a Neubauer haemocytometer, whilst growth was expressed as relative growth rate (K) using the equation provided by Levasseur et al.28
K' = Ln (N2/N1)/ (t2-t1)
Where Ln is the natural log, N1 and N2 are the cell concentrations (cells.mL-1) at time1 (t1) and time2 (t2) respectively.
The procedure to determine the oil content of an algal biomass was adopted from Lee et al. 29. The biomass was harvested from the culture medium by centrifugation at 8,000 rpm for 10 minutes. About 120 mg biomass (dry weight) was used for lipid extraction. The cells were suspended in 5 mL of phosphate buffer (pH 7.4) and treated in a bead-beater (KCM Catering Equipment, model HBB908, Hamilton Beach, Richmond, Virginia) for 1 minute using 1 mm glass beads. The ruptured cells were transferred to a separation funnel and 30mL of chloroform: methanol (2:1v/v) was added for lipid extraction. The mixture containing algal cells was shaken vigorously for 20 minutes and left to stand for 30 minutes. After phase separation, the organic layer was decanted. The residual cells were treated with 20 mL solvent once again and the organic layer was recovered. The combined chloroform/ethanol extract was washed with 20 mL 5 % (w/v) sodium chloride solution. The solvent was evaporated from the extract at room temperature under a closed fume hood. The total lipids were measured gravimetrically. The oil content of the sample was reported as extract weight/dry biomass. The oil content in each group of algae was extracted from the species which showed highest cell concentration/mL at the peak exponential growth phase. In this case, D. tertiolecta showed the highest growth rates in the given culture period; biomass of this alga was therefore collected and oil extracted so that it would function as something of a representative of the Chlorophyta algal division. Similarly, N. oculata was chosen from the Chrysophyta division and P. tricornutum was chosen as a representative of the Bacillariophyta. Growth responses of the algae were calculated and expressed as the average percentage change in cell concentration from the inoculated concentration and results graphed accordingly. Results were statistically analysed with one and two-way ANOVA using Microsoft Excel 2010 (Seattle, Washington) and statements of significance refer to the 0.05 level.
The comparative water chemistry of coastal seawater and inland saline groundwater from the Victorian goundwater monitoring bore 116677, located in Koonda, Victoria, 3669, Australia, at the salinity level of 19.6 ppt, is demonstrated in Table 1.
|
Major ions |
Diluted coastal seawater (mg.l-1) |
Inland saline groundwater (mg.l-1) |
Concentration equivalence of inland saline groundwater to diluted coastal water |
|
Chloride |
11305 |
11470 |
1.01 |
|
Sodium |
5944 |
4960 |
0.83 |
|
Sulphate |
1789 |
1000 |
0.56 |
|
Magnesium |
1245 |
2139 |
1.72 |
|
Potassium |
232 |
20 |
0.09 |
|
Calcium |
118 |
289 |
2.45 |
|
Phosphate as Phosphorus |
27 |
<0.05 |
|
|
Fluoride |
0.39 |
0.13 |
0.33 |
|
Nitrate as Nitrogen |
<0.20 |
<0.20 |
N/A |
|
Ammonia as Nitrogen |
0.18 |
0.06 |
0.33 |
|
Nitrite as Nitrogen |
0.01 |
0.02 |
2 |
Table 1 Comparative water chemistry of coastal seawater and inland saline ground water that featured as the focus of this study
The cell density of all three Chlorophyta microalgal species increased rapidly and reached maximum numbers between days 10-14 with initial apparent lag phase (Figure 1). In all three species from the division Chlorophyta, D. tertiolecta biomass increased more than that of the other two species (T. suecica and N. atomus) at a significant level (P-value of 0.025, > α = 0.05). For Chrysophyte algae, while there were gradual increases in the cell density during the corresponding period, the growth observed was generally less than that of Chlorophyte algae (Figure 2). Interestingly, the growth of Chrysophyte algae followed a similar trend to that of the Chlorophytes in that all three species reached their maximum cell densities during days 10-14; nonetheless, the growth pattern demonstrated that there are no significant differences between each of the species being tested (P-value of 0.159, > α = 0.05). However, between days 10 and 14, the increase in cell density of N. oculata, was higher than that of I. galbana (T.iso) and P. lutheri.
The cell density of Bacillariophyta algae did not follow the trend of the Chlorophytes and Chrysophytes (Figure 3). The cell density of all three species of Bacillariophyta algae decreased considerably from day 8 until the end of the experimental period, though P. tricornutum appeared to perform considerably better than the other two Bacillariophyta species (N. closterium and C. fusiformis). The species-wise variation in cell densities in the Bacillariophyta algae was significant (Figure 3) (P-value of 0.020, > α = 0.05). The increase in cell density of P. tricornutum was relatively rapid, with increases from 26.38 × 104 (at day 0) to 121.63 × 104 cells. ml-1 (at day 14) and decreased to 68.33 × 104 cells. ml-1 by day 20.
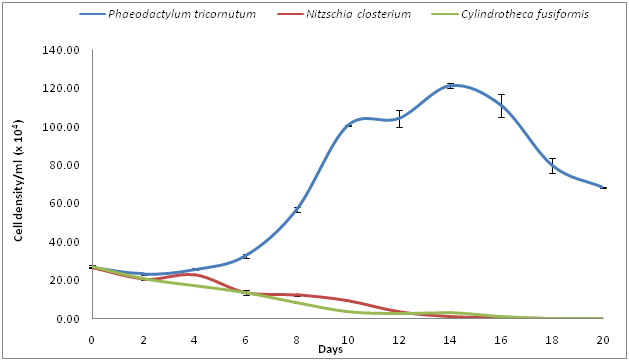
Figure 3 Growth observed in cell density (cells.ml -1) for different species of Bacillariophyte algae.
During the corresponding period, the cell densities of N. closterium and C. fusiformis decreased sharply and the growth rate became negative. Towards the end of the experimental period, the cell density decreased to 0.023 x 104 and 0.005 x 104 cells ml-1 for N. closterium and C. fusiformis, respectively. The growth rates of the Chlorophyte algae cultures are depicted in Figure 4. At the beginning, the growth rate of all three species increased. The highest growth rate was demonstrated by D.tertiolecta (1.169), followed by T. suecica (1.161). After day 15, the growth rate of this group started decreasing and a negative growth rate was recorded on day 16, which continued right up until day 20. On the other hand, the low growth rate of all algal species of Chrysophyte algae continued to day 14, but decreased considerably thereafter (Figure 5). The highest growth rate was shown by N. atomus, (1.091) followed by P. lutheri (0.840) and Isochrysis sp (0.811). All the species of diatoms (Bacillariophytes) showed poor growth rates (Figure 6), exhibiting negative growth rates from the beginning, except the species P. tricornutum, which showed a positive growth rate (the highest was 0.569 ) and only commenced a negative growth rate on day 16, which continued right up until day 20. N. closterium and C. fusiformis did not demonstrate any positive growth during the entire culture period. The oil content in the D. tertiolecta biomass harvested during the stationary phase was about 37.4 % (w/w, dry basis). Oil content in N. oculata was found to be within the normal range (34%) and oil content in P. tricornutum was relatively very low (4% dry weight) (Figure 7).
The growth patterns of all three divisions of microalgae studied in inland saline ground water (ISGW) were found to be different. There were several differences in the ionic compositions in the ISGW compared to coastal sea water at the same salinity level. ISGW contained greater concentrations of magnesium and calcium, though was very low in potassium, phosphate and ammonia-nitrogen. On the other hand, diluted coastal water was low in sulphate but was high in potassium, phosphate and nitrate-nitrogen. The ionic compositions of the two types of water that were investigated as part of this study were probably responsible for the observed growth differences in the microalgae that were studied. Inland saline waters in Australia have a similar composition to seawater, in that they are dominated by sodium and chloride ions, but inland saline waters are not fully congruent with seawater in the concentrations of other ionic components. Some groundwater, even saline groundwater, may have more bicarbonate (HCO3-) than sulphate (SO4++) and more silicon (Si) than potassium (K+). All of the elements required for algae growth are present in the oceans, in low concentrations that are not toxic, do not interfere with each other and are present in forms that can be actively (and passively) concentrated to meet the needs of the plant. This is not always the case with groundwater’s, which may have toxic concentrations of some elements and insufficient concentrations of others.19,22 It is likely that silica could have been the limiting factor for the growth of diatoms, since this element is required for construction and maintenance of the “skeletons” of these organisms.30-32
As stated by Cordover .13 the loss of dissolved potassium in the saline ground waters of the Murray Darling Basin results in a serious deficiency of that element, with concentrations often only 15% of that in seawater at the equivalent salinity. Potassium is an important electrolyte used to replace sodium in cell metabolism and has been found to be a critical constraint to terrestrial plants in saline soils.33 Potassium has a specific role in plant metabolism as an enzyme activator .34 and very low potassium concentrations in inland saline waters would no doubt result in poor seaweed (macroalgal) growth, since potassium is one of the most common salts in seawater .35 It could therefore reasonably be suspected that low potassium concentrations that featured in the ISGW in this study could have hampered and retarded the growth of some of the microalgal species that featured in this study.
Potassium and magnesium have both been suggested as being key ions for the physiological functioning and growth of fish and shrimp.36 and are sometimes deficient in many brackish or low salinity environments throughout the world.37,38 It has been suggested that simply increasing potassium concentrations to levels that would be expected in dilute seawater, along with slight increases in magnesium and sulphate, can help to solve problems, such as mortality, associated with low salinity aquaculture.37,39 It is noteworthy that the same could be true for waters that are suitable for the culture of microalgae, particularly with regards to increases in the levels of potassium.
Chlorophytes had higher biomass productivity, specific growth rate and lipid content than those demonstrated by Chrysophytes and Bacillariophytes in this study. It is very likely that the physiological stress caused by an excess of nutrients, presence of metals and possibly other organic compounds present in ISGW generated the lower results achieved in the Chrysophyta and Bacillariophyta. However, according to Wood et al.40 the physiological adjustments necessary for a strain of microalgae to be considered acclimated are only achieved after successive transfers in the stressful medium and several generations after the initial inoculums, where efficient adaptation of the microalgae is achieved for the new growing conditions. Algae transferred from algal stock cultures of natural sea water at 33.6% salinity to experimental culture mediums with salinities of 19.6% would have been subject to osmotic stress with direct impact on the cellular water potential (which would have increased with an influx of the hyposmotic water), whilst algae transferred into ISGW would have also had to adapt to the change of the cellular ionic ratios.41 It is unknown how large the effect of the change in cellular ionic ratios would have affected the cultures in the ISGW, but it is expected to have extended the lag phase and therefore decreased the potential maximum cellular density and growth velocity of cultures. Some algae are able to withstand extreme conditions of salinity, temperature and contaminants, having a competitive advantage over other more sensitive microalgae. Salt stress is one of the many environmental factors that limit growth and productivity of microorganisms.
Salinity can be a major factor in the retardation of central metabolic activities such as photosynthesis in plants.42 Microalgal species differ in their adaptability to salinity and can be categorized as being halophilic and halotolerant based on their salinity tolerance level.43 In natural conditions or marine waters, active multiplication typically starts at day 5 or 7 of the growth phase. Variations in salinity also influence several biochemical and physiological mechanisms such as lipid production and growth which are essential in marine organisms.44 Species of Dunaliella are very well adapted to propagate in media ranging from far less than seawater salt concentrations (in fact, virtually able to withstand exposure to freshwater with a salinity level of 0.5%) to saturated, hypersaline salt solutions (with a salinity level of 292.2%).42 This extremely halotolerant member of the Chlorophyta, being able to withstand such extreme conditions, performed very well and demonstrated very good growth in this study, which should hardly be considered surprising owing to its ability to tolerate such extreme conditions, thus conferring an advantage to this species of microalgae, which should be remembered when selecting a species for commercial application(s).
Tetraselmis spp. are renowned as a relatively very adaptable algal species and have been shown to be capable of growth at salinity levels varying from brackish (20%) up to hypersaline (50%), with maximum biomass reported at 40%.45.,46 though it must be reported that the decreasing biomass yields reported by these same authors may have resulted due to the non-adaptability of the organism to higher salinity.47 Overall, it must be said that Tetraselmis did not perform particularly well in this particular study, but a logical extension of the current study would be to investigate further the effects of different strains of this algae after two to three generations, as has been mentioned previously, thus adding a dimension of robustness to our results.
The influence of different salinities and concentrations of dissolved nutrients (N and P) in the growth rate of N. oculata was studied by Arriada & Abreu.6 The authors observed that, despite this species of microalgae being highly adaptable to environmental variations; its optimal growth is governed by the interaction of temperature and salinity, which have been show to exert influence over the nutritional properties of microalgae.48
There are several possible reasons for the relatively very poor growth of diatoms, but the most likely are low salinity, ion imbalances and physiological stress brought upon by a lack of silica. Saros & Fritz.49 showed that diatom physiology can be affected directly or indirectly via interaction with other growth factors such as the ion composition in a saline system. In a few species of diatoms, low salinity can result in a decrease of cell dimensions 50 In this study, P. tricornutum appeared to be an exception and performed considerably better than the other two species of diatoms. P. tricornutum presumably has tolerance to a wide range of salinities, which is consistent with work that has been conducted in the coastal and offshore waters of Korea.51-53 One of the limitations of this study was that no silica detection was done in the ISGW, either in quantity, or quality, as would be necessary for diatoms. Neither was any information obtained regarding the silica level of the seawater, as is typically required by marine hatcheries. Silica is arguably the most important part of diatom biology and ecology. The weight of silica influences the cell's float/sink equilibrium and influences cell size and accumulation of photosynthetic storage products. The effects of different external conditions (light, temperature, nutrients, trace metals) lead to different ratios of Si:N and Si:P and also affect carbon metabolism (Si:C, N:C, P:C) differentiall.30-32,54-56 A major consequence of Si limitation for diatoms is that the internal reserves of most species are not sufficient to allow maintenance or construction of the cell wall as the organism grows, thus inhibiting cell division and disallowing the sustenance of long periods of growth.57-59 Because silicon is such an important part of the skeletal matrix of diatoms, it could reasonably be expected that most of the silicon inside the cell is going to be concentrated within the cell wall, or else complexed with organic compounds or perhaps sequestered in vesicles rather than being free in solution, meaning that growth models cannot be directly linked to the total cellular Si quota.60 Indeed, Harrison et al.54 found that diatom growth was inhibited more from Si starvation than under conditions of reduced nitrogen or phosphorus concentration. This could readily explain why the diatoms in this study demonstrated negative growth.
The oil content of various algae strains may vary significantly depending on the growth conditions and stage of the algae in question.61 The oil content in D. tertiolecta biomass harvested during the stationary phase was about 37.4 % (w/w, dry basis) which was within the range of D. tertiolecta oil content as reported in the literature (36-42 % dry weight;62. The oil content in N. oculata (34%) was found to be within the typical range of that reported in the literature (31-68 % dry weight) .9 However, the oil content in P. tricornutum was very low (4% dry weight), which is considerably below the typical range (which is 31% dry weight; Sheehan et al.9 Yet another indication that the diatom species that were being studied were physiologically stressed was the very limited (or sometimes, zero) oil content that was present in the case of N. closterium and C. fusiformis.
Microalgae cultivation in ISGW still has enormous potential despite the negative growth of two diatom species and the comparatively slow growth of Chrysophyte algae in this study. The results have to be interpreted with some caution because the suitability of microalgae cultivation in inland ground saline water was based only on the increase in cellular concentration and lipid contents and without detecting all levels of macro and micronutrients available in the used water. More controlled research on different groups of microalgae would be a good investment and a synergistic approach is recommended, including water chemistry, hydro-geology, soil-water interactions of and nutritional profiles of microalgae in ISGW. The linkage between water resources and primary production (including analysis of water quality and nutrient chemistry) should be continued for each site, since water characteristics differ according to different aquifers and across different soil types. The concentration of dissolved minerals and their dynamics needs to be monitored both in situ and under laboratory conditions so that sustainable microalgae growth can be optimised in the field.
The study successfully demonstrated that the Chlorophytes group of marine microalgae can be successfully grown in inland saline groundwater. Onshore cultivation of marine seaweeds is becoming more and more popular because of the limited availability of nearshore sites suitable for algal farming and because of the high cost of building and maintaining structures for these farms in the sea. Although many existing farms are near the sea, there is no reason why microalgae farming could not be located inland near a suitable saline ground water source. This study has examined the capacity of ISGW to be a viable and alternative medium in the production of marine microalgae of different groups for aquaculture and non-aquaculture industries.
None.
None.

©2015 Awal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.