Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 4
Department of Botany, Magway University, Myanmar
Correspondence: Kyi Kyi Myint, Associate Professor, Department of Botany, Magway University, Myanmar
Received: July 06, 2019 | Published: July 25, 2019
Citation: Myint KK, Nwe MM, Mar TL. Study on morphological characters of some mangrove plants in South-eastern Ayeyarwady Delta of Myanmar. J Aquac Mar Biol. 2019;8(4):118-128 DOI: 10.15406/jamb.2019.08.00250
A study on the mangrvoe plants in Pyapon Township, Ayeyarwady Region, South-eastern Ayeyarwady Delta (between Lat. 94˚30′ and 95˚45′ North and between Long. 15˚30′ and 16˚25′ East), Myanmar was conducted within the period of March 2016 to February 2017. A total of 18 species of mangroves plants were recorded in the natural mangrove areas. In the present study, the taxonomic descriptions of mangrove plants were presented.
Keywords: taxonomic, Ayeyarwady Delta
'Mangrove' has been variously defined in literature. The oxford dictionary mentioned the words 'mangrove' and 'mangrowe' since 1613, indicating tropical trees or shrubs found in coastal swamps with tangled roots that grow above the ground, whereas the Americans, the Spanish, and the Portuguese used the term 'Mangle' and 'Mangue' indicating trees and shrubs of the genus Rhizophora.1 Later, the term 'mangrove' was referred to the individual plant or tidal forest or both, as 'Mangrove plants' and 'Mangrove ecosystem'.2 In addition, mangroves have been defined by Hamilton and Snedaker3 as salt tolerant ecosystems of the intertidal regions along coastlines. Moreover, the term ‘mangrove’ is used to define both the plants that occur in tidal forests, and to describe the community itself.4,5 Mangrove plants mostly grow within the sheltered intertidal flat deltaic lands, funnel-shaped bays, broad estuarine mouths, shallow or frequently tidal inundated coast lines.6
Mangrove forests were of crucial significance for local people, providing food, shelter and, medicinal and other uses. The local people depend on mangroves for fire-wood, charcoal, timber, poles and many other purposes. Traditional uses of mangrove resources currently continue side by side with large-scale and intensive exploitation using high-capital investment and technologies such as charcoal production and firewood. In recent years, efforts to convert mangrove land for other uses, such as fish- or shrimp-ponds or industrial and human settlements have been increasing in number and size. Nevertheless, mangrove provided directly and indirectly uses in various ways.
The mangrove forests provide livelihood and employment to wood cutters, fishermen, honey and wax collector, shell collectors, timber traders and their workers and other peoples who work both seasonally and round the year in the forest.
Mangroves occur in 112 countries and global coverage has been estimated at over 179,000km2,710million hectares,8 14-15million hectares9 and 24million hectares,10 more than 18million ha.4 A study by Giri et al.11 estimated that the total mangrove forest area of the world in 2000 was 137,760km2 with the largest extent of mangroves found in Asia (42%) followed by Africa (20%), North and Central America (15%), Oceania (12%) and South America (11%). The Sundarbans, the mangrove forest in Bangladesh, is the largest mangrove forest in the world. Australia has the third largest area of mangroves in the world. India is one of the 15 most mangrove-rich countries having 2.7 % of global total area of mangroves. Asia contains most of the world’s mangroves with 46%.12 The largest areas of mangrove in Southeast Asia are found in Indonesia (almost 60 % Southeast Asia’s total), Malaysia (11.7%), Myanmar (8.8%), Papua New Guinea (8.7%) and Thailand (5.0%).
Globally, there are 110 recognized species of plants classified as mangroves, belonging to 20 different families.13 However, Tomlinson4 reported that around 34 major and 20 minor mangrove species belonging to about 20 genera in over 11 families have been recorded globally.
It is well known that Southeast Asia’s mangroves are the best developed and probably the most species-diverse in the world. Giesen & Wulffraat, 1998 reported that 52 species are found in the Southeast Asian. Wang et al.14 reported that 59 species in 41 genera and 29 families of mangrove plants were recorded in India and 26 species of 12 families and 15 genera were from China. Hening15 recorded 69 species under 34 families in the whole of Sundarban mangrove. However, Karim16 reported 123 plant species belonging to 22 families representing 30 genera in Bangladesh. Santisuk17 reported that 74 species under 53 genera were recorded in Thailand.
Myanmar is endowed with a rich diversity of habitat types arising largely from its unusual ecological diversity. Myanmar has more than 2,000kilometres of coastline along the Bay of Bengal. Generally, it comprises the Rakhine, Ayeyarwady Delta and Tanintharyi region, where mangroves are common. About 8.8percent of Southeast Asia’s mangroves are located in Myanmar, of which 46 percent is located in Ayeyarwady (Irrawaddy) Division, 37 percent in Tanintharyi Division and 17 percent in Rakhine State. The largest mangrove areas were observed in Ayeyarwady Delta having 180,826 ha compared to other areas of Rakhine (64,777ha) and Tanintharyi (140,485ha). Mangrove forests are mainly found in Bogalay, Pyapon and Laputta townships, which are situated in the south of the Ayeyarwady Delta.18 The total area was about 530,000 hectares in 1980, but it decreased to 425,000 hectares by 2000, or perhaps as low as 382,032ha.
The various authors studied the marphological characters of mangrove plants along the various localities of Myanmar coastal regions byThan Htay and Saw Han,19 Aung Myint & Kyaw Soe,20 Cherry Aung,21 San Tha Tun,22 Tin Hla and Htun Paw Oo,23 Thi Thi Htaik,24 Mar Lar,25 San Tha Tun,26 Nyo Nyo San.27 The purpose of this study is to elucidate the distribution, occurrence and taxonomic descriptions of mangroves.
Study area: The natural mangrove formations were observed along the Tebinzeik creek and Thaung-ga-done creek that located between Htaung Gyi Tan and Ah Shey Hpyar villages and War Kone village. War Kone village is situated at the inner part of Ahmar Sub-Township. These villages were located the south-eastern Ayeyarwady Delta, Ayeyarwady Region, Pyapon Township (between latitudes 94˚30′ and 95˚45′ North and between longitudes 15˚30′ and 16˚25′ East), Myanmar.
Observation of Mangrove plants in the field: In this study, mangrove plants were collected from March 2016 to February 2017. The morphology of mangroves were recorded from the natural mangrove areas. Taxonomic descriptions were described in the fieldwork and floral parts such as flowers, leaves were taken to the laboratory to make herbarium sheets and preserved.To study the morphology of the mangroves, twenty transects were assigned perpendicular to the creek crossing through the forest from seaward to landward. Permanent plots (10m×10m) were set along each transect. T1–T7 were set from near the Htaung Gyi Tan village, T8–T16 from Ah Shey Hpyar and T17– T20 from War Gone villages respectively. The location of the transect lines along the creek of mangrove areas were shown in the Figure 1.
In the present study18 species of mangroves that belong to 11 families and 13 genera have been recorded from the natural mangrove areas of Htaung Gyi Tan, Ah Shey Hpyar and War Gone villages, Pyapon Township, South-eastern Ayeyarwady Delta. The recorded plants included 14 species of woody trees, 2 species of shrubs, 1 species of monocot plams and 1 species of fern.
The classified list of mangrvoe plants were mentioned in Table 1 and the morphological descriptions were also presented.
|
Division |
Class |
Order |
Family |
Genus |
Species |
Local name |
Type |
1 |
Magnoliphyta |
Magnoliopsida |
Scorphulariales |
Acanthaceae |
Acanthus |
Acanthus volubilis |
Khayar-phyu |
Shrub |
2 |
|
Liliopsida |
Arecales |
Arecaceae |
Nypa |
Nypa fruticans |
Dani |
Palm |
3 |
|
Magnoliopsida |
Lamiales |
Avicenniaceae |
Avicennia |
Avicennia marina |
Thame-phyu, Thame-ywet-leit |
Tree |
4 |
|
|
|
|
|
Avicennia officinalis |
Thame-gyi, Thame-net, Thame-ywet-wyne |
Tree |
5 |
|
|
Euphorbiales |
Euphorbiaceae |
Excoecaria |
Excoecaria agallocha |
Tayaw, Thayaw |
Tree |
6 |
|
|
Sapindales |
Meliaceae |
Xylocarpus |
Xylocarpus moluccensis |
Kyana, Kyat-nan |
Tree |
7 |
|
|
Primulales |
Myrsinaceae |
Aegiceras |
Aegiceras corniculatum |
Yae-kha-yar |
Tree |
8 |
|
|
Plumbaginales |
Plumbaginaceae |
Aegialitis |
Aegialitis rotundifolia |
Pinle-sar, Sar-thar- pin |
Shrub |
9 |
Polypodiophyta |
Polypodiopsida |
Pteridiales |
Pteridaceae |
Acrostichum |
Acrostichum aureum |
Hnget-gyi-taung |
Fern |
10 |
Magnoliphyta |
Magnoliopsida |
Rhizophorales |
Rhizophoraceae |
Bruguiera |
Bruguiera cylindrica |
Hnan-byu |
Tree |
11 |
|
|
|
|
|
Bruguiera gymnorrhiza |
Byu-oak-saung, |
Tree |
12 |
|
|
|
|
|
Bruguiera sexangula |
Byu-shwe-war |
Tree |
13 |
|
|
|
|
Ceriops |
Ceriops tagal |
Madama-myaw |
Tree |
14 |
|
|
|
|
Rhizophora |
Rhizophora apiculata |
Byu-chidauk-ahpho, Byu-chidauk-ywet-chun |
Tree |
15 |
|
|
Myrtales |
Sonneratiaceae |
Sonneratia |
Sonneratia alba |
Lame |
Tree |
16 |
|
|
|
|
|
Sonneratia apetala |
Kambala |
Tree |
17 |
|
|
|
|
|
Sonneratia griffithii |
Laba |
Tree |
18 |
|
|
Malvales |
Sterculiaceae |
Heritiera |
Heritiera fomes |
Pinle-kanazo , Yae-kanazo |
Tree |
Table 1 Classified list of mangrove plants
Acanthus volubilis Vahl.
Syn: Dilivaria ebracteata Vers.
Shrubs, upto 1.5m height. Stem solid, glabrous, terete. Leaves simple, opposite and decussate, exstipulate; petiole 0.5-0.7cm long and 0.3-0.4cm broad, solid, glabrous, pulvinous; lamina oblong to elliptic-oblong, 10.0-12.0cm long and 4.0-5.0cm broad, the base angled, the margin spiny to very spiny and wavy, the tip acute. Inflorescence raceme, spike 11.0-16.0cm long, peduncled. Flowers 5.5-6.0cm long and 3.3-3.5cm across; bract 1, 1.5-1.8cm long and 0.8-1.0cm broad, curved, ovoid, entire, acute, basal cup-shaped, glabrous, greenish yellow; 2 bracteoles partly covered by bract, smaller than bract; showy flower, erect, sessile, bisexual, zygomorphic, complete, hypogynous. Sepals 4, aposepalous, 2 outer one larger, 2 inner one smaller, ovate, 1.7-2.0cm long and 0.8-1.1cm broad, acute, basal curved, coriaceous, glabrous, greenish, persistent. Petals 1, ovate, 3.5-4.0cm long and 2.5-3.0cm broad, entire, obtuse, coriaceous, slightly fleshy, slightly curved, margin outer folded, large, showy, white, deciduous. Stamens 4, free, filament about 1.9cm long and about 0.25cm broad, fleshy, solid, white, glabrous, flattened, erect; anther dithecous, about 0.8cm long, basifixed, densely white, pubescent, longitudinal dehiscence, inserted, introse. Carpels 2, syncarpous, ovary dumble shaped, 0.2-0.3cm long, glabrous, terete, two locules, two ovules in each locule, ovary superior, axile placentation, style terminal, persistent, glabrous, white, stigma bifid. Fruit capsule, 2.5-3.0cm long and 0.8-1.0cm in diameter, glabrous. Seeds 0.7-1.0 cm long, kidney shaped, grey (Figure 2).
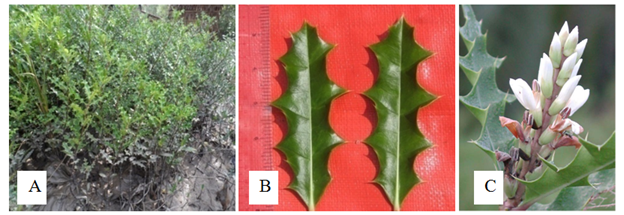
Figure 2 Acanthus volubilis: (A) Habit. (B) Leaves and (C) Inflorescences 2. Nypa fruticans Wurmb.1781.
Flowering and fruting periods– May to August
Palms, 3.5-4.5m height, dichotomously branched, rhizomatous stems. Leaves spirally arranged, pinnately compound, upto 5m long, shiny, glabrous, green, rachis stout, sheathing base. Inflorescence spadix on a long peduncle, upto 50.0cm long, several spathe enclosed flowers, peduncle erect, stout, glabrous, green. Male flowers gregariously on each peduncle, covered by spathe; tepals 6, apotepalous in two whorls, ovate elliptic, upto 0.8cm long and 0.5cm broad, entire, acute, tufted, glabrous, shiny; stamens 3, united to form central column, anthers dithecous, upto 0.4cm long. Female flowers enclosed by spathe, tepals 6; capels 3, funnel shaped, upto 1.0cm height, angular, unilocular, single ovule, basal placentation, style and stigma 0. Fruit globose. Seeds broadly ovoid (Figure 3).
Flowering and fruting periods– November to July
Avicennia marina (Forsk.) Vierh. 1907.
Syn: Sceura marina Forsk.1775.
Rach bruce Trav. 1790.
Racka torrid J.F.Gmel.1791.
Halodendron thourasi Roem. & Schult. 1818.
Racka ovate Roem. & Shult. In L. 1818.
Trees, up to 10m height; pencil-like pneumatophores, arising upward from the horizontal spreading roots. Leaves opposite and decussate, exstipulate, simple; petiole 1.0-1.3cm long, slightly flattened, glabrous, pulvinous; lamina ovate-lanceolate, 8.0-10.0cm long and 2.5-3.5cm broad, glabrous, the base cuneate or tapering, the margin entire, the tip acute, coriaceous, the upper surface shinning and the lower surface much silvery or white, pubescent. Inflorescence condensed, terminal or axillary cyme, globose, compound spike, infrequently branched, glabrous, peduncle long, up to 10 cm. Flowers 0.5-0.9cm long and 0.4-0.5cm across, sessile, orange yellow, bracteate, bracteolate, complete, regular, hypogynous. Sepals 4, aposepalous, elliptic, 0.25-0.35cm long and 0.15-0.2cm broad, entire, blunt, coriaceous, persistent, outer surface hairy, tomentose, imbricate. Petals 4, synpetalous, the tube short, th lobe 4, ovate, 0.3-0.4cm long, 0.25-0.3cm wide, acute, orange yellow, hairy outside and glabrous within, valvate. Stamens 4, about 0.2cm long, epipetalous, filament round, white, glabrous; anther dithecous, yelloe, basifixed, longitudinal dehiscence, extrorse, exserted. Carpel 3, ovary 0.2-0.3cm long, oblong, style terminal, short, stigma 2, bi-fid, glabrous below, hairy above. Fruit capsule, compressed, shortly straight beak (Figure 4).

Figure 4 Avicennia marina: (A) Habit. (B) Stem base and its pneumatophores. (C) Stem. (D) Leaves (E) Inflorescence and (F) Fruits.
Flowering and fruting periods– March to October
Avicennia officinalis L.1753.
Syn: Anacardium orientale Houst.1662.
Oepata rheed Hort.1683.
Avicennia tomemtosa Willd. 1800.
Avicennia oepata Hamilt. 1835.
Medium to tall trees, 15-20m height; pneumatophores numerous, long, spongy, pencil like erect. Leaves opposite and decussate, simple, exstipulate; petiole 1.3-1.5cm; lamina obovate-oblong, 9.5-10.5cm long and 5.0-5.8cm broad, the margin entire, obtuse or rounded apex, green, leathery not much silvery beneath, glabrous. Inflorescence compound spike, with 10-12 flowers in each branch, capitate globose, panicles, up to 7.8cm long, peduncle up to 24.0cm long. Flowers 0.4-1.1cm long and 1.1-1.5cm broad at anthesis; bracts 2, small with fimbriate margin; bracteoles 2; sessile, complete, bisexual, dull yellow, actinomorphic, hypogynous. Sepals 4, aposepalous, elliptic, 0.5-0.6cm long and 0.25-0.3cm broad, entire, acute, coriaceous, pubescent, quincuncial, persistent. Petals 4, apopetalous, elliptic, 0.5-0.8cm long and 0.5-0.6cm broad, entire, acute, curved in the tips, quincuncial, orange yellow, deciduous. Stamens 4, apostemonous, alternate, 0.3-0.4cm long, filaments 0.2-0.25cm long, round, glabrous; anther dithecous,0.1-0.15cm long, yellow, basifixed, extrorse, exserted, longitudinal dehiscent. Carpel elliptic or slightly oblique, 0.65-0.7cm long, with shortly hairs, 1 locule, style 1, 0.15-0.75cm long, glabrous, stigma capitate. Fruit fleshy, ovoid, 3.5-5.0cm long and 2.4-2.8cm broad, densely hairy, flattened, wrinkled at the base and with a short narrow beak. Seed single, dark green (Figure 5).

Figure 5 Avicennia officinalis: (A) Habit. (B) Stem base and its pneumatophores. (C) Stem. (D) Leaves. (E) Inflorescence and (F) Fruits.
Flowering and fruting periods– March to October
Excoecaria agallocha L.1759.
Syn: E. camettia Willd.
E.affinis Endl.
Stillingia agallocha Baill.
Medium trees, up to 15.0m height, with poisonous milky latex; root without any aerial growth. Stems round, solid. Leaves spirally arranged, simple, exstipulate, occasionally reddish; petiole 1.5-2.0cm in length and 3.0-3.5cm broad, glabrous; lamina ovate elliptic, 5.0-7.0cm in length and 2.5-3.5cm broad. Male inflorescence catkins, peduncle 5.0-7.0cm long, deciduous, bracteate, sessile, dioecious, small, odorless; tepals 3, ovate, 0.12-0.15cm long, apotepalous, irregularly acute, yellow, small, valvate, persistent; stamens 3, free, filament 1.2-1.3cm long, glabrous, anther dithecous, about 0.1cm long, basixed, extrorse, exserted, longitudinal dehiscence. Female inflorescence mixed cyme, bracteate, glandular, sessile, bracteoles pair, round, glabrous, dioecious; tepals 3, ovate, apotepalous, valvate, green, glabrous, acute, small, persistent, odorless; Carpel 3, syncarpous, superior, ovary ovoid, 3 locule, basal placentation, one ovule in each locule, stlyes 3, curved, simple, about 0.2cm long, stigma 3, terminally bent, slightly pubescent. Fruits 3-lobed, schizocarp, about 0.6cm in diameter, dehiscing into 3 cocci to release the solitary seeds. Seeds black, about 6.0mm in diameter (Figure 6).

Figure 6 Excoecaria agallocha: (A) Habit. (B) Stem base. (C) Stem. (D) Leaves. (E) Male inflorescence. (F) Male flowers. (G) Female flowers and (H) Fruit.
Flowering and fruting periods – May to August
Xylocarpus moluccensis (Lamk.) M. Roem.1879.
Syn: Carapa molluccensis Lam.
C. indica Juss.
Medium trees, up to 10m height, buttresses absent, conical like pneumatophores. Stems rough, erect, solid, woody, longitudinal fissured. Leaves paripinnately compound, leaflets 4-6, exstipulate; petiole 0.5-0.7cm long, dark brown, glabrous, pulvinous; lamina ovate-elliptic, 10.0-13.5cm long and 5.0-7.0 cm width, entire, slightly mucronate, upper surface dark green, lower surface whitish, glabrous, coriaceous. Inflorescence united cymes, 7.0-7.5cm long, with distinct main axis. Flowers tetramerous, 0.6-0.65cm long and about 0.3cm across; pedicel about 0.5cm long, glabrous, brown. Sepal 4, aposepalous, ovate, about 0.15cm long and about 0.2cm broad, green, blunt, entire, coriaceous, glabrous, valvate, deciduous. Petal 4, apopetalous, ovate, about 0.3 cm long and about 0.25cm broad, thin, white, slightly curved, odourless, twisted, deciduous. Stamens 8, fuse to form globose tube, staminal tube present, 8 apical short lobe; lobe ovate, about 0.15cm long; anther dithecous, inserted, introrse, dorsifixed, longitudinal dehiscence, inferior. Carpel 8, syncarpous; ovary globose, about 0.2cm in diameter; 8-locules, two ovules in each locule, axile placentation, style terminal, about 0.1 cm long, gradually tapering, glabrous; stigma 1, flat. Fruits round spherical or globose, with long woody stalk, fruit coad woody, pendulous and hanging on branches, 12.0-15.0cm across, 4 sutures of woody valves, well filled with 15-20 seeds. Seeds angular look like pyramidal, tetrahedral, hard (Figure 7).

Figure 7 Xylocarpus moluccensis: (A) Habit. (B) Stem base. (C) Pneumatophores. (D) Leaves. (E) Inflorescence. (F) Fruit and (G) Seeds.
Flowering and fruting periods– March to September
Aegiceras corniculatum (L.) Blanco.1837.
Syn: Rhizophora corniculatum L. 1754.
Aegiceras majus Gaertn. 1788.
Small tree, up to 4m height. Stems solid, cylindrical. Leaves alternate, simple, exstipulate, petiole 0.8-0.9cm long, 0.2-0.3cm width, glabrous, pulvinous; lamina obovate to oblong, 8.4-8.7cm length and 4.0-4.2cm broad, the base cuneate, the margin entire, the tip round to emerginate, shiny, coriaceous. Inflorescence simple umbel, terminal. Flowers 1.2-1.4cm long and 0.8-1.0cm in across; bracts very minute, deciduous, triangle in shaped; pedicel 0.6-0.7cm long and 0.1cm width, glabrous; ebracteolate, complete, bisexual, regular, actinomorphic, pentamerous, twisted, hypogynous, fragrance. Sepals 5, aposepalous, 0.4cm long and 0.3cm broad, round apex, curved, coriaceous, twisted, sepaloid, persistent, inferior. Petals 5, synpetalous, about 1.0cm long and 1.0-1.2cm broad, white, reflexed, entire, acuminate, twisted. Stamens 5, opposite to petal, filaments 0.4cm long, base united to form a tube, anthers dithecous, sagittate, creamy white, exserted, introrse, dorsifixed, longitudinal dehiscence, inferior. Carpel 1, monocarpelly, about 0.3cm long, 2 ovules in each locule, free central placentation, style 1, terminal, about 0.7cm long, little red sports in the middle of style, glabrous, soft, stigma absent, superior. Fruits capsule, 6.0-8.0cm long, completely curved with pointed apex, calyx persistent at the base. One seeded, 1.0-1.1cm long (Figure 8).

Figure 8 Aegiceras corniculatum: (A) Habit. (B) Stembase. (C) Leaves (D) Inflorescence (E) Close up view of inflorescence. (F) Flowers and (G) Fruits .
Flowering and fruting periods– January to September
Aegialitis rotundifolia Roxb.1824.
Syn: A.annulata Kz.
Shrubs, up to 3m height, without any aerial roots, latex present, mucilage. Stems cylindrical, base swollen look like buttresses, branches more or less spongy with leaf scars. Leaves alternate, simple, exstipulate, petioles 9.0-10.0cm long and 0.5-0.7cm in diameter, tubular leaf-sheath, glabrous; lamina broadly ovate, 9.0-9.2cm long and 7.0-7.5cm broad, the upper surface shiny, slightly fleshy, dark green, the base cuneate, the margin entire, the tip obtuse. Infloresecence raceme, peduncle 6.5-7.0cm long, developed from sheathing petiole. Flowers 1.5-1.8cm long and about 1.0cm in across; bract ovate-lanceolate, 1.0-1.2 cm long and 0.5-0.7cm broad; pedicel 0.3-0.4cm long, terete; bracteoles 0.6-0.8cm long, entire, obtuse, mucronate apex, curved, green; complete, bisexual, regular, actinomorphic, pentamerous, hypogynous. Clyx 5, synsepalous, slightly connate at base, lobe 1.0cm long and 0.2 cm broad, entire, acute, glabrous, coriaceous, green, valvate. Petals 5, apopetalous, linear oblong, 11.0-12cm long and 0.15-0.2cm wide, entire, obtuse, thin, white, glabrous, odourless, imbricate. Stamens 5, slightly connate at the base, short stamina tube, 0.3-0.5cm long; filament about 1.3cm long, equal in length, exserted, white, glabrous; anther dithecous, sagittate, about 0.2cm long, yellowish, basifixed, longitudinal dehiscence, extrose. Carpel 5, syncarpous, ovary oblong, about 0.3cm long, one locule, basal placentation, style 5, terminal, about 0.4cm long, glabrous, white. Fruit capsule, 6.0-8.0cm long, with persistent calyx, linear. Seeds 6.0-7.0cm long, black (Figure 9).

Figure 9 Aegialitis rotundifolia: (A) Habit. (B) Stem base. (C) Leaves. (D) Inflorescence. (E) Flowers and (F) Fruits.
Flowering and fruting periods – Aprial to October
Acrostichum aureum L.
Erect fern, attaining 1-2m in height, small bushy appearance; rhizomatous with fibrous like roots. Frond simple, stout, erect, 1.0m long and 4.0cm width, covered with large scales. Tips of fertile frond rusty-brown, maturing dark brown during spore release, tips of sterile frond blunt with a short tip, unicostate reticulate venation, glabrous, coriaceous. Mature frond become sporophyllous, diffuse sporangia at abaxial surface, mixed sporangia on both mid vein, brown sporangia stalked, upper globose (Figure 10).

Figure 10 Acrostichum aureum: (A) Habit. (B) Fertile fronds. (C) Leaves. (D) Rhizome and (E) Circinate young fronds.
Sporangia formation – November to February
Bruguiea cylindrica (L.) Blume. 1827.
Syn: Rhizophora cylindrica L.1753.
Rhizophora caryophylloides Burm. f. 1768.
Bruguiera caryophylloides Blume, Enum.1827.
Large tree, upto 20.0m height; pneumatophores knee like and buttresses forms. Stem glabrous, solid. Leaves simple, opposite and decussate, exstipulate, petiole 2.0-2.3 cm long, pulvinous; lamina ovate-lanceolate, elliptic, 13.8-14.0 cm long and 4.8-5.2cm broad, the margin entire, the tip acute, glabrous, coriaceous. Inflorescence cymose, 3-flowers in each peduncle, peduncle long, terete, green, glabrous. Flowers 1.0-1.3cm long and 0.3-0.5cm in across, ebracteate, pedicel 0.2-0.3cm long, glabrous, smooth, pale green, ebracteolate, complete, bisexual, regular, actinomorphic, epigynous, creamy white in colour. Sepals 8, synsepalous, 0.4-0.5cm long and 0.2cm broad, surface smooth, pointed apex, entire, valvate, thick, hard, persistent. Petals 8, aposepalous, 0.35-0.4cm long and 0.15-0.2cm broad, each petal folding, two parts, thin, terminal small ciliated and basal hairy along the margin. Stamens16, free, 8 in groups, 2 stamens in each petal, filaments unequal, about 0.15-0.3cm long, introrse, inserted, anther dithecous, 0.15-0.2cm long, basifixed, longitudinal dehiscence. Pistils cup-shaped, ovary 0.15-0.2cm long, 2-4 celled, one ovule in each locule, axile placentation, style terminal, 0.75-0.9cm long, glabrous, stigmas bifid, hairy. Fruit capsule, pendulous, persistent calyx, the lobes bent towards the pedicels (Figure 11).
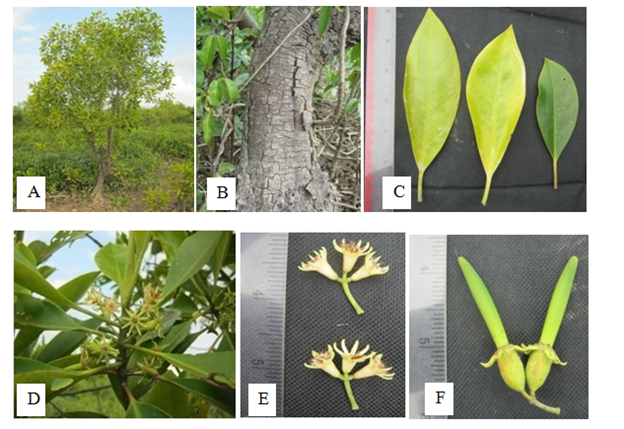
Figure 11 Bruguiea cylindrica: (A) Habit. (B) Stem. (C) Leaves. (D) Inflorescences. (E) Flowers and (F) Fruits.
Flowering and fruting periods– March to November
Bruguiera gymnorrhiza (L.) Lamk.1797-98.
Syn: B. conjugate (L.) Merr.
B. rheedii Miq.
Rhizophora gymnorhiza Roxb.
Everygreen tree, up to 10m height; aerial roots from knee like pneumatophores developing from bending horizontal underground roots; root buttress thickened the trunk base. Stem solid. Leaves opposite and decussate, simple; petiole 3.5-4.5cm long, glabrous, reddish, solid, pulvinous; lamina broadly ovate-lanceolate, 16.0-19.0cm long and 5.0-6.5cm broad, reddish distinct mid-vein in the leaves, the margin entire, the tip cute, coriaceous, shiny, glabrous. Inflorescence axillary, solitary, large, cymose. Flower pendulous, elliptical, 3.0-3.5cm long and 2.5-3.5cm broad at anthesis, ebracteate; pedicel about 2.0cm long, round, glabrous, reddish; ebracteolate, complete, bisexual, regular, actinomorphic, cyclic, epigynous. Sepals 13-16, aposepalous, about 1.3cm long, with distinct ribbed, reddish, conspicuously fleshy lobes, apicular, apex pointed, hard, persistent, valvate. Petals 13-16, apopetalous, about 1.0 cm long and about 0.2cm broad, oblong, coriaceous, reddish, upper surface slippery, terminally 4 cilia, white, 0.3-0.4cm long. Stamens 26-32, free, two stamens pair within one petal, pair stamens with unequal filament; filament up to 0.3cm long, glabrous; anther about 0.25cm long, dithecous, basifixed, longitudinal dehiscence, introrse, inserted. Pistil cup-shaped, 2-4 locules, 2 ovules in each locule, style terminal, about 0.7cm long, glabrous, stigma 3-lobed, glabrous. Fruit capsule, pendulous with persistent calyx, one-seeded (Figure 12).
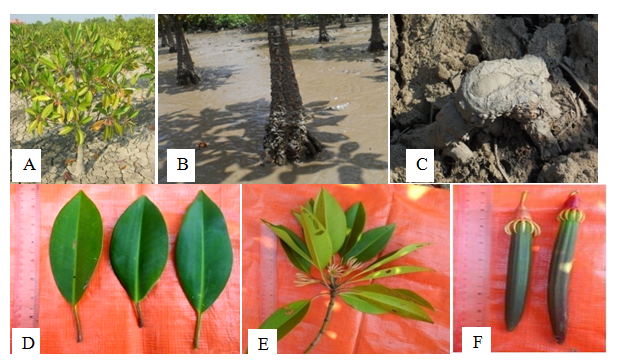
Figure 12 Bruguiera gymnorrhiza: (A) Habit. (B) Stem. (C) Knee root. (D) Leaves. (E) Inflorescences and (F) Fruits.
Flowering and fruting periods– March to October
Bruguiera sexangula (Lour.) Poir. Lamk. 1816.
Syn: B. eriopetala W. & A.
B. rumphii Bl.
B. parietosa Griff.
Small trees, up to 5-10m height; aerial roots from root buttresses and developed significantly the conical trunk base or broom like trunk with stilt roots base when young and knee like pneumatophores. Stems solid. Leaves opposite and decussate, simple; petiole 2.8-3.0cm long, glabrous, greenish yellow, solid, pulvinous; lamina ovate-lanceolate, 11.5-13.5cm long and 4.5-5.6cm broad, glabrous, yellowish distinct mid-vein, the upper surface shiny, the margin entire, the tip acute, coriaceous, fleshy, stipulate. Inflorescence axillary, solitary, large, cymose. Flower large, pendulous, 3.8-4.0cm long, ebracteate; pedicel 0.8-0.9cm long, curved, terete, solid, smooth, glabrous, yellowish, pendulous, ebracteolate, complete, bisexual, regular, actinomorphic, cyclic, epigynous. Sepals 10-12, synsepalous, sepal tube 1.5cm long, sepal lobe 2cm long, with distinct ribbed, yellowish, conspicuously fleshy lobes, apicular, apex pointed, hard, persistent, valvate. Petals 10-12, apopetalous, 0.7-0.8cm long, bilobed, folding into one, each lobe 0.18-0.2cm broad, lanceolate, terminally not ciliated, coriaceous, tufted, basal side pubecent, reddish brown, glabrous, outer surface slippery, pale red inner surface, slightly rought. Stamens 24-26, free, 10-12 groups, 2 stamens into each folding petal, unequal filaments, up to 0.7cm long, the base of filament hairy, white, inserted, introrse; anther dithecous, 0.38-0.4cm long, basifixed, longitudinal dehiscence, yellow. Pistil cup-shaped, ovary 0.4-0.5cm long, 2-4 celled, 2 ovules in each locule, axile placentation, style terminal, 0.8-1.0cm long, white, glabrous, stigma 3, small, ovate, glabrous, entire, fleshy. Fruits indehiscent pod, pendulous, green, slightly angular (Figure 13).

Figure 13 Bruguiera sexangula: (A) Habit. (B) Stem base. (C) Leaves. (D) Inflorescences. (E) Flowers and (F) Fruits.
Flowering and fruting periods – March to October
Ceriops tagal (Perr.) C. B. Robison. 1908.
Syn: Rhizophora tagal Perr. 1824.
Ceripos candolleana Arn., Ann.1838.
Small to moderate trees, 5.0-8.0m height; aerial roots broom like and form buttresses on trunk base. Stems solid. Leaves opposite and decussate, simple; petiole 1.8-2.0cm long and 0.2cm broad, glabrous, flattened, pulvinous; lamina elliptic, 9-10cm long and 5.8cm broad, the base obtuse, the margin entire, apex rounded, coriaceous, glabrous. Inflorescence cymose, axillary, flowers cluster, on long slender peduncle, peduncle 0.9-1.0cm long, glabrous, flattened. Flowers pentamerous, 0.5-0.6cm long and 0.4-0.45cm in diameter, bisexual, complete, regular; pedicel short, 0.1cm long, glabrous, minute. Sepals 5, aposepalous, elliptic-lanceolate, 0.4-0.45cm long and 0.2-0.25cm broad, entire, thick, green, acute, persistent. Petals 5, apopetalous, oblong, 0.25-0.3cm long, entire, thin, reddish brown, thick, terminally 3-clavate appendages present, valvate. Stamens 10, free, filament 0.25-0.28cm long, unequal, 5 short and 5 long, reddish brown; anthers dithecous, sagittate, 0.1cm long, shorter than the filament length, light brown, dorsifixed, introrse, inserted, longitudinally dehiscence. Carpel 3, syncarpous, inferior, elliptic globose, 0.15-0.18cm long, axile placentation, 2 locules, 2 ovules in each locule, style terminal, 0.2-0.3cm long, reddish brown, glabrous, stigma 3, shortly tri-fid. Fruits indehiscent, 20-26cm long, with 5 persistent calyx, green to yellowish brown, surface warty, ridged and grooved, hanging and pointed. Seed single (Figure 14).

Figure 14 Ceriops tagal: (A) Habit. (B) Leaf. (C) Fruit. (D) Inflorescence as seen. (E) Inflorensence and (F) Flower.
Flowering and fruting periods– February to September
Rhizophora apiculata Blume. 1827.
Syn: Rhizophora candellaria DC. 1828.
Rhizophora conjugate Sensu. Arn. 1859.
Medium trees, up to 12 m height. Silt roots, looping from lower branches and trunk base. Stem solid, glabrous; bark grey to dark grey, longitudinally fissured. Leaves opposite and decussate, simple, exstipulate; petiole 2.6-2.8cm long and 0.35-0.4cm broad, slightly flattened, glabrous, green, pulvinous; lamina ovate-elliptic, 14.0-14.3cm long and 6.4-7.0cm broad, the margin entire, the tip acute, coriaceous. Inflorescence cymose, 2.5-2.9cm long, 2-flowered on each peduncle, peduncle 0.8-1.0cm long and 0.5-0.7cm diameter, glabrous. Flowers 1.2-1.4cm long, ebracteate, sessile, complete, bisexual, regular, actinomorphic, glabrous, complete, regular, odorless, globose in bud, medium. Sepals 4, aposepalous, ovate, 1.2-1.6cm long, 0.9-1.0cm broad, entire, acute, fleshy, thick, glabrous, valvate, persistent. Petals 4, apopetalous, lanceolate, 0.8-1.0 cm long and 0.2-0.25cm broad, entire, acute, glabrous, fleshy, deciduous, odourless. Stamens 11-12, free, sessile, anther dithecous, sagittate, 1.0-1.2cm long, introrse, inserted, longitudinal dehiscence, white. Carpels 2, syncarpous, ovary globose, 0.2-0.25cm long, glabrous, 2 locules, 2 ovules in each locule, axile placentation, style terminal, very short, stigma bifurcate. Fruit capsule, 45.0-47.0cm long and 1.5-2.0cm in diameter, tapering towards the apex, with 4 persistent sepals. Seeds 1, 1.5-1.7cm long and 2.0cm in dameter (Figure 15).
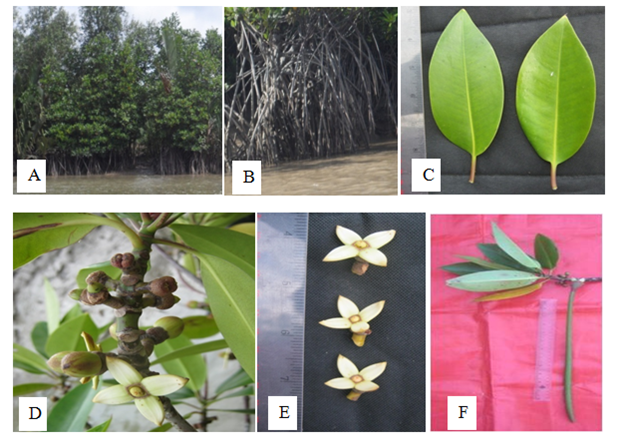
Figure 15 Rhizophora apiculata: (A) Habit. (B) Stilt roots. (C) Leaves. (D) Inflorescences. (E) Flowers and (F) Fruit.
Flowering and fruting periods– March to September
Sonneratia alba J. Smith. 1879.
Syn: S. mossambicensis Klotz.
S. acida Benth.
Tree, up to 10m height; bark pale brown to deep brown, smooth with fine longitudinal fissures; pencil like pneumatophores. Leaves simple, opposite and decussate, exstipulate; petiole 0.5-1.0cm long; lamina obovate-elliptic to almost round, 7.5-8.0cm long and 4.0-5.0cm broad, margin entire, the tip blunt, glabrous, coriaceous, fleshy. Inflorescence terminal, few flowered cyme. Flowers 6.5-7.0cm and 7.0-8.5cm in across at anthesis, ebracteate, pedicel 4.0-5.5cm long, glabrous, complete, bisexual, regular, actinomorphic, perigynous. Calyx 6, synsepalous, the tube cup-shaped, with 6 lobes, oblong-elliptic, 2.5-4.0cm long and 1.0-1.5cm broad, green outside and red or purple inside, linear, entire, acute, glabrous, fleshy, coriaceous, valvate, persistent. Petals 6, polypetalous, linear, upto 3.5 cm long and upto 0.5cm broad, truncate, entire, acuminate, white, glabrous, valvate, deciduous, glabrous. Stamens numerous, free, showy, filament 4.0-4.5cm long, white, glabrous; anther dithecous, 0.1cm in diameter, yellow, dorsifixed, exserted, extrorse, longitudinal dehiscence. Carpel 6, syncarpous, ovary cup-shaped, 2.0-2.5cm long and 4.0-4.5cm in diameter, shining, flattened, round, 20 locules, numerous ovules in each locule, axile placentation, style terminal, 5.0-6.0cm long, glabrous, persistent,stigma capitate, 0.5-0.2cm in diameter, glabrous. Fruits globose-berry, 5.0-8.0cm in diameter, lower part somewhat enclosed in turbinate calyx, at maturity flat and with sharp pointed, long style. Seeds numerous (Figure 16).
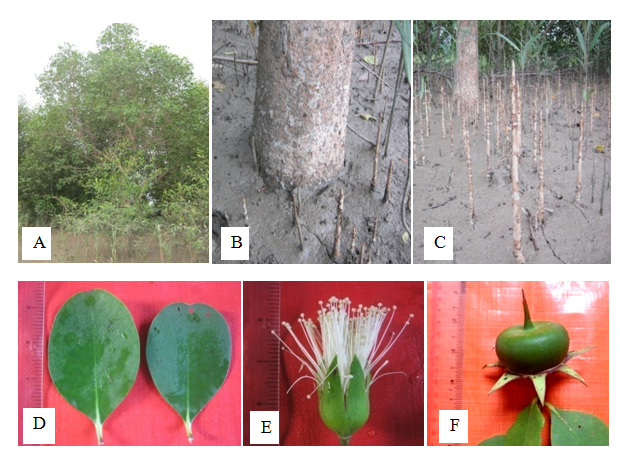
Figure 16 Sonneratia alba; (A) Habit. (B) Stem base. (C) Pneumatophores. (D) Leaves. (E) Flower and (F) Fruit.
Flowering and fruting periods– April to October
Sonneratia apetala Buch. 1800
Syn: Kambala apetala Rafin. Sylb. Tell. 1838.
Blatti apetala O. K. Rev. Gen. 1891.
Trees, up to 20m height, drooping twings and branchlets; pencil like pneumatophores present, tapering the ends. Stem irregularly fissured. Leaves opposite and decussate, simple, exstipulate; petiole about 0.6cm long, glabrous, pulvinous; lamina oblong-lanceolate, narrow, gradually tapering towards the apex, 8.0-12.0cm long and 2.0-3.0cm wide, glabrous, shiny, the base attenuated, the margin entire, the tip obtuse. Inflorescences terminal or axillary, mostly 3 flowered, dichasial cyme. Flowers 1.7-3.0cm long and 2.5-3.0cm in across, bracterate; pedicel 1.8-2.0cm long; complete, bisexual, regular, actinomorphic, tetramerous, hypogynous, yellowish white. Calyx 4, synsepalous, the tube cup-shped, the lobes elliptic, 1.5-1.7cm long and 0.7-0.8cm broad, entire, acute, valvate, persistent. Petals absent. Stamens many, free, filament 1.0 cm long, creamy yellowish white, glabrous, inserted, anther dithecous, pale yellow, 1.0cm long, dorsifixed, longitudinally dehiscence. Carpel 4, syncarpous, ovary dumble shaped, 0.4-0.5cm long and 0.6-0.8cm in diameter, superior, 5 locules, numerous ovules in each locule, axile placentation, style 1,terminal, glabrous, yellowish white, 1.5-2.0cm long, curved, stigma 1, lage and umbrella-shaped. Fruits globose, berry, 2.0-2.2cm in diameter, depressed, arranged on the flattened persistent calyx. Seeds 1.4cm long, brown, triangular, ridged (Figure 17).
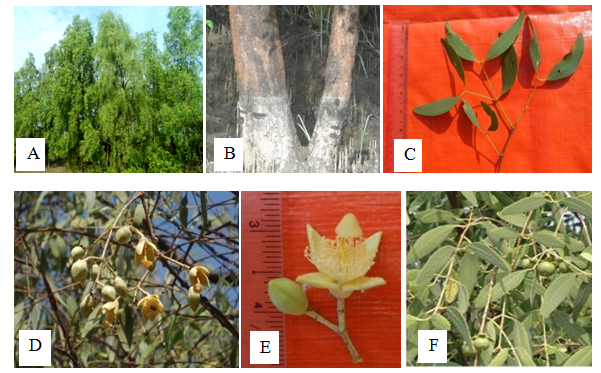
Figure 17 Sonneratia apetala: (A) Habit (B) Stem base and its pneumatophores. (C) Leaves. (D) Inflorescence as seen. (E) Flower and (F) Fruits.
Flowering and fruting periods – June to October17. Sonneratia griffithii Kurz. 1871.
Sonneratia griffithii Kurz. 1871.
Syn: Sonneratiaalba Griff.
Tree, upto 15m height; pencil like pneumatophores with tapering end. Stem terete, glabrous, solid; bark cream to brown, with longitudinal fissures. Leaves simple, opposite and decussate, leathery and exstipulate; petiole 0.1-0.3cm long, glabrous, pulvinous; lamina obovate or broadly oval, 13.0-13.3cm long and 11.0-11.3cm wide, the base very broadly round or subcordate, the margin entire, the tip round, fleshy, glabrous, shiny. Inflorescence terminal, cymose. Flowers 3.0-4.5cm long and 5.0-6.8cm across, ebracteate, pedicel upto1.0cm long, glabrous, ebracteolate, incomplete, bisexual, actinomorphic and perigynous. Sepals 6, synsepalous, the tubes cup-shaped, the lobes lanceolate-elliptic, 2.0-4.0cm long and 0.5-1.0cm wide, entire, acute, sepaloid, fleshy, slightly flattened, valvate, persistent. Petals absent. Stamens numerous, free, filaments 2.0-3.5cm long, white, soon shed following anthesis, glabrous; anther dithecous, basifixed, exserted, white, introrse, longitudinal dehiscence. Carpals 6, syncarpous, ovary globose, 2.0-3.0cm long and 4.0-5.0cm in diameter, green, glabrous, multilocular, numerous ovules in each locule, axile placentation; style 1, terminal, 3.0-4.0cm long, soft, terete, glabrous, persistent; stigma 1, globose. Fruit is globose berry, 5.0-7.0cm in diameter, terminally into short style beak, partly enclosed by peristent green sepals at base. Seeds numerous, angular, light brown (Figure 18).
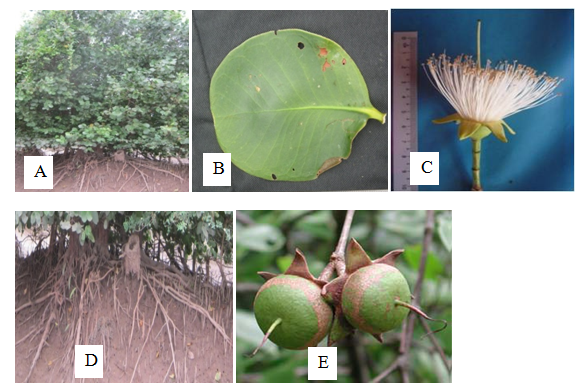
Figure 18 Sonneratia griffithii: (A) Habit. (B) Leaf. (C) Flower. (D) Pneumatophores. and (E) Fruits.
Flowering and fruting periods– April to October
Heritiera fomes Bunch. Ham.1800.
Syn: Heritiera minor Roxb.1832.
Every green trees, up to 10m height; stem base buttress, blunt end peg-like pneumatophores present. Leaves alternate, simple, stipules in pair in each node; petioles about 1.0 cm long, terete, grey, slightly pulvinous; lamina elliptic-lanceolate, 8.0-14cm long and 3.0-4.5cm wide, the margin entire, the tip acute, tapering, coriaceous, slightly hard, dorsal surface pale green, glabrous, ventral surface whitish grey, rough. Inflorescence mixed; peduncle 5.5-6.0cm long, bent and pendulous, much branched. Flowers 1.0cm long and 0.5cm across, small, ebracterate, regular, unisexual, odourless; pedicel about 0.5cm long, pubescent, soft. Calyx 4-5, synsepalous, calyx tube cup-shaped, 0.3-0.35cm long, outer surface orange-yellow, inner surface reddish-orange towards the base, imbricate, inferior. Petals absent. Male flowers: stamens 5, fused to form pistillode; anther encircling the apical portion of the pistillode, to form globose, dithecous with apical white beak, yellow, longitudinal dehiscence, introrse, inserted. Female flowers: carpels loosely attached, 0.2-0.25cm long, each ovary flattened, globose, pubescent, one ovule in each locule, basal placentation; style terminal, about 0.1cm long, white; stigma pentafid. Fruit capsule, sub-globose, 1.5-2.0cm long, up to 5.0 cm in diameter, pericarp brown, hard, woody divided into 2 halves by distinct ridged, 1-seeded (Figure 19).

Figure 19 Heritiera fomes: (A) Habit. (B) Stem. (C) Pneumatophores. (D) Leaves. (E) Inflorescence and (F) Fruit as seen.
Flowering and fruting periods– May to September
Myanmar has more than 2,000kilometres of coastline along the Bay of Bengal. Generally, it comprises the Rakhine, Ayeyarwady Delta and Tanintharyi Region, where mangroves are common. Ayeyarwady Delta is in the southern part of Myanmar. It is located between latitudes 15° and 18° north, and between longitudes 94° and 96° east. The total area of Ayeyarwady Region (including the whole Ayeyarwady Delta) is approximately 155,795km2. The mangrove of Ayeyarwady Delta is the largest having 180,826 ha, compared to other areas of Rakhine (64,777 ha) and Tanintharyi (140,485 ha) in Myanmar. In Ayeyarwady Delta, mangrove forests are mainly found in Bogalay and Laputta Townships, which are situated at southern part of Ayeyarwady Delta.18
According to San Tha Tun,26 a total of 41 species of mangroves includes trees, shrubs, herbs, lianas, palms and fern were recorded along the U-To tidal creek, Chaung Tha, western part of Ayeyarwady Division. He reported that Rhizophora apiculata, R. mucronata, Bruguiera gymnorhiza,and Ceriops decandra were common and Bruguiera sexangula, B. parviflora and Ceriops tagal were rare in Chaung Tha area, western part of Ayeyarwady Region. In construct to this data, Bruguiera sexangula species was abundant in the present study area but this species is very rare in Chaung Tha. Likewise, Ceriops decandra was very rare in the present study area but this species is common in Chaung Tha.
The composition of mangroves plants from the natural mangrove forests near the study area were also recorded. A total of 18 species of mangrove plants were recorded in the present study. The common mangrove species are Bruguiera sexangula, Avicennia officinalis, Ceriops tagal and Excoecaria agallocha in the areas.Among the families, Rhizophoraceae, Rhizophora apiculata, Bruguiera sexangula, B.gymnorhiza and Ceriops tagal are more common and Rhizophora mucronata and Ceriops decandra are very rare in the present study.
In the present study, mangrove plants have been recorded from the natural mangrove areas of Htaung Gyi Tan, Ah Shey Hpyar and War Gone villages, Pyapon Township, South-eastern Ayeyarwady Delta, in Myanmar. In the present study, Avicennia officinalis, Bruguierasexangula and Excoecaria agallocha are the most common in the natural mangroves of South-eastern Ayeyarwady Delta. Now this mangrove areas are gradually declined because local people are clear cut the mangrove trees for fairwood, charcoal and other uses. They haveto work two opportunities to stand their life, fishing and cutting the trees. Nowadays, government, other NGOs and local people are united to estiblish the restored mangrove areas.
I would like to acknowledge my indebtedness to Dr Khin Mg Oo, Rector of University of Magway and Dr Soe Win and Dr Than Than Oo, Pro-Rectors of University of Magway for their kind permission to carry out this research. I am deeply grateful to Dr Aye Aye Kyi, Professor of Department of Botany, University of Magway for providing facilities for laboratory apparatus, her permission and understanding me through present work.I would like to acknowledge my deepest respect and indebtedness to supervisor Dr. Htay Aung, (Now, Retired and dead) Rector of Mawlamyine University for his valuable guidance, criticism and suggestion and for his kind permission to carry out this research work.I am indebted to Dr Khin Maung Cho, Professor and Head of Department of Marine Science, Pathein University (Now retired) for his useful advice and critical suggestions. I would also like to acknowledge Dr Myint Myint Cho, Professor, Department of Marine Science, Mawlamyime University for her valuable criticism and advice. I am also dreadful to U Aung Myint, Director of Renewable Energy Association Myanmar (REAM) and U Saw Han Shein, Rector of Mawlamyine University (Retired) for their valuable advices, constructive criticisms and facilities of the books. I also especially want to thank U Kyi Lin, Staff Officer, U Kyaw Zaw, Range Officer, U Tin Bo, U Han Myo Linn, U Tin Maung Htwe, Rangers, U Kyaw Naing, Forest Guard and other staff members, Ministry of Environmental Conservation and Forestry, Pyapon Township, Ayeyarwady Region, for their assistance during my field works. Finally, I would like to express my gratitude to my parentfor their encouragement and understanding to accomplish this research.
The author declares that there are no conflicts of interest.

©2019 Myint, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.