Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 3
1Department of Marine Sciences, University of South Alabama, USA
2Industrial Phycology, Bristol, UK
3Department of Oceanography, Dalhousie University, Canada
Correspondence: Lucie Novoveská, Department of Marine Sciences, University of South Alabama, USA
Received: April 29, 2019 | Published: May 7, 2019
Citation: Novoveská L, MacIntyre HL. Study of the seasonality and hydrology as drivers of phytoplankton abundance and composition in a shallow estuary, Weeks Bay, Alabama (USA). J Aquac Mar Biol. 2019;8(3):69-80. DOI: 10.15406/jamb.2019.08.00245
Small, shallow estuaries can be highly vulnerable to land use changes, eutrophication and habitat loss but are understudied with respect to their larger counterparts. Where they are monitored, the descriptors of their environmental status are typically chlorophyll a as a proxy for phytoplankton abundance and nutrient concentration as a presumed driver of the phytoplankton community. We present data from a shallow estuary, Weeks Bay, Alabama (USA), that demonstrates that chlorophyll a and nutrient concentrations are inadequate descriptors of ecological state. Weeks Bay had relatively high nutrient concentrations (86–169µM total nitrogen and 1.0–5.2µM total phosphorus) and highly variable chlorophyll a concentrations (2.2–160.5μgL-1). The variability in chlorophyll a was most highly correlated with nutrient levels and river discharge. There was no relationship between chlorophyll a and community composition. Two of three maxima in chlorophyll a (> 100 μgL-1) were caused by non-toxic chlorophytes and diatoms; the third was dominated by potentially toxic raphidophyte Heterosigma akashiwo. The phytoplankton were diverse even at the class level and community composition varied on both annual and inter-annual scales. The best overall descriptor of phytoplankton composition was the annual cycle in temperature, but inter-annual variability was correlated with hydrology. In the winter, dominance by dinoflagellates, including several taxa that form harmful algal blooms, was correlated with low river discharge, low turbidity and high zooplankton numbers, while dominance by diatoms was correlated with high and variable river discharge and high turbidity. In the summer, dominance by cryptophytes versus diatoms was consistent with changes in groundwater discharge. The dominance of harmful algal bloom taxa vs non-toxic ones could not be inferred from chlorophyll a and/or nutrient concentrations.
Keywords: phytoplankton, composition, chlorophyll, estuary, variability
Phytoplankton play a crucial role in the global ecosystem, contributing to a significant portion of primary production.1,2 While they support marine food-webs, certain species can also form deleterious harmful algal blooms (HABs) or, under eutrophic conditions, result in detrimental ecosystem outcomes such as hypoxia or the loss of submerged aquatic vegetation.3–5 Shifts in community composition and the factors that underlie them have been studied extensively in the context of HABs because of their ecological and economic costs. Generally, phytoplankton community composition is determined by the availability of nutrients and light, temperature, salinity, physical losses such as sinking and flushing, biotic interactions such as grazing, competition and viral lysis, and susceptibility to toxins and allelopathic compounds.6 It is a specific combination of the above factors, at a given point in time that enables one or a few species to dominate over others, and ultimately shape the community.7 Humans directly or indirectly affect all of these factors.
Although nutrient cycling has received much attention, the ability of a population to accumulate in an ecosystem depends on the relative rates of growth and losses, including flushing.8 Variation in river discharge alters hydraulic residence time but can also influence the concentration and chemical species of nutrients, the stability of the water column and water clarity. The majority of studies describing the effect of discharge and transport on marine phytoplankton biomass and composition have focused on large estuaries such as San Francisco and Chesapeake,9–11 river plumes12–14 or coastal systems such as the Baltic Sea15 that have relatively long residence times of several months. Shallow sub-tropical estuaries have received less attention, with the exception of a small number of well-studied systems such as Thau Lagoon, France16 and Indian River Lagoon, USA17,18 which also have relatively long residence times. In contrast, there is very little information on the dynamics of phytoplankton community composition in small estuaries with short residence times, particularly those influenced by submarine groundwater discharge.
The goal of this study was to assess the appropriate descriptors of ecological status in a shallow, tropical estuary, Weeks Bay, Alabama (USA). The bay has been the site of dense HABs of the dinoflagellatesKarlodinium veneficum, Prorocentrum minimum, Heterocapsa triquetra and Kryptoperidinium foliaceum and the raphidophytes Chattonella subsalsa and Heterosigma spp. (unpublished data). There was a bloom of Heterosigma during the study but dominance by HAB taxa was correlated with neither chlorophyll a (Chla) nor nutrient concentrations. We show that the annual temperature cycle and hydrology are major drivers of both phytoplankton biomass and community composition. Neither detection not prediction of HABs could be achieved by monitoring Chla and nutrients alone.
Weeks Bay is a shallow (<2m deep) sub-estuary 7km2 area located between the Fish and Magnolia Rivers and Mobile Bay (Figure 1). Mobile Bay is the second-largest estuary in the northern Gulf of Mexico after the Mississippi. The Fish River watershed encompasses 143km2 and contributes approximately 70% of the surface freshwater flow to Weeks Bay.19 The remaining discharge to Weeks Bay comes from the Magnolia River, located to the east. The residence time under average discharge conditions is 13 days, with a range from 0.5 to 100 days.19 Tides in Weeks Bay are diurnal and have a mean range of 0-0.4m.19 Nutrient inputs to Weeks Bay originate in river discharge (primarily from the Fish River), groundwater and benthic fluxes.20,21 The Fish and Magnolia Rivers are the surface expression of the aquifer in a single hydrological unit.22 Surface runoff from agricultural and urban land alters nutrient concentrations in the discharge23,24 and the aquifer is contaminated with c. 2mM nitrate adjacent to Weeks Bay.25 The sediment can be net heterotrophic and a sink for nutrients but benthic productivity throughout Weeks Bay is generally high (annual mean 0.25gCm-2d-1)26 and limited by light rather than nutrient availability.27 Sediments in the mouth of Fish River and Weeks Bay were identified as a sink for nitrate and a source for phosphate and ammonium, although there is high temporal and spatial variability in the fluxes.21 Benthic fluxes may therefore contribute substantially to the nutrient budget.

Figure 1 Map of the Weeks Bay watershed, Alabama, USA, showing the sampling site () (30 24.97’N, 87 49.37’W), locations of NOAA’s meteorological station (□) maintained by Weeks Bay National Estuarine Research Reserve, the USGS groundwater well (▲), and USGS discharge gage on Fish River (●).
The study site (30 24.97’N, 87 49.37’W) is located at the mouth of Fish River, where the mean water depth is 2m. This site has coarser sediments than most of Weeks Bay proper, where the sediment type ranges between sand and silt. The salinity is relatively low at the study site, averaging 6, but higher values are common during dry episodes. Land-use in the watershed is classified as agricultural, forested and residential.28 However, the human population of Baldwin County, in which Weeks Bay is located, grew by 19.6% between 2010 and 201829 and it continues to increase. Land-use is residential and forested immediately adjacent to the study site.
Data collection
Surface water samples were collected at the study site biweekly from August 26, 2008 to August 24, 2010. The sampling site was located at the end of the pier at the mouth of Fish River and northern end of Weeks Bay estuary (Figure 1). Each sample was collected from the same location in the early afternoon (13:00±1 hour). The physical parameters, temperature, salinity, dissolved oxygen (DO) and turbidity, were measured at the surface and bottom of the water column, using a calibrated sensor (556 Multiprobe System, YSI, Yellow Springs, OH). The sample was split into three acid-washed polycarbonate or glass bottles at the time of collection. The first bottle was put on ice immediately for nutrient and HPLC pigment analysis. The second bottle was preserved with Lugol’s acidic solution for microscopic observations of preserved microalgae and the third bottle was kept for observation of live cells. Both the second and third bottle were kept at ambient temperature. Water samples were always processed within 3 hours of collection.
Separate data on water depth, turbidity and dissolved oxygen (DO) at the study site were obtained from a mooring operated by the National Oceanic and Atmospheric Administration (NOAA) as part of the System Wide Monitoring Program (SWMP). The mooring (Figure 1) is maintained by the Weeks Bay National Estuarine Research Reserve (NERR) for the Centralized Data Management Office30 and records temperature, salinity (as conductivity), pH, dissolved oxygen, turbidity and depth every 15minutes. Data on wind velocity and direction and total flux of photosynthetically available radiation (PAR) was collected by the Safe Harbor meteorological station (supported and maintained by the same agencies), which is located less than 1km from this site.30 Discharge data were obtained from the US Geological Survey gauging station 02378500, which is 19km upstream in Fish River (Figure 1).31 The depth of the water table (distance to the groundwater) was obtained from USGS water well 302416087505501, which is located on the north margin of Weeks Bay about 1.8 km west of the sample site.32
Laboratory analyses
Samples were filtered through Whatman GF/F filters. The filtrate was analyzed for inorganic nutrients (NO3-, NO2-, NH4+, PO43-, SiO32-) which were determined directly by colorimetric techniques using a Skalar SAN+ nutrient autoanalyzer.33 The level of detection (concentration of blank plus 3xstandard deviation of the blank) were 0.08µM for NO3-, 0.02µM for NO2-, 0.17µM for NH4+, 0.05µM for PO43-, and 0.11µM for SiO32-. Particulate phosphorus (PP), total phosphorus (TP), and total dissolved nitrogen (TDN) concentrations were measured after oxidation to orthophosphate or nitrate.34,35 Particulate carbon (PC) and nitrogen (PN) were measured in the material collected on pre-combusted GF/F filters using a Costech CNS analyzer. Dissolved inorganic nitrogen (DIN) was calculated as the sum of NO3-, NO2-, NH4+. Dissolved organic nitrogen (DON) was calculated as the difference between TDN and DIN. Dissolved organic phosphorus (DOP) was calculated as the difference between TP and sum of PP and DIP. Total nitrogen (TN) and total phosphorus (TP) are the sum of dissolved and particulate pools.
Phytoplankton composition and abundance
Sub-surface phytoplankton densities were determined using an OM900 inverted light microscope (Meiji Techno America, Santa Clara, CA) with a highest magnification of 400X. Diatoms, dinoflagellates, chlorophytes, prasinophytes, euglenophytes, cryptophytes, raphidophytes, dictyophytes and cyanobacteria were enumerated in a Nunc settling chamber (Thermo Fisher Scientific, Orchester, NY) according to Tomas.36 Diatoms and dinoflagellates were identified to genus/species where possible using standard texts.36 A minimum of 200 phytoplankton cells was counted. Zooplankton were not identified to genus/species and only the total abundance of all zooplankton (including ciliates and various larvae) was recorded simultaneously with phytoplankton densities.
Pigment concentrations
Concentrations of chlorophylls and carotenoids were measured by high performance liquid chromatography (HPLC) using an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA), with a Zorbax Eclipse C8 column (4.6x150 mm, 3.5μm) and quantified against authenticated standards (DHI Lab, Horshølm, Denmark). Samples were stored at -80°C until analysis. The pigments were extracted in 90% v:v acetone (Baker Analyzed HPLC solvent), sonicated and filtered through 0.45μm syringe filters (SUN-SRi, HPLC filter, PTFE, membrane) prior to injection.37
Statistical analysis
Phytoplankton community composition was analyzed using Primer-E statistical software (Primer-E v6, Plymouth, UK). Seasons were defined in terms of trimesters: winter: Dec, Jan, Feb; spring: Mar, April, May; summer: Jun, July, Aug; fall: Sep, Oct, Nov. Cell abundances were square-root transformed to reduce the influence of the most common species in between-sample comparisons. Non-parametric, multivariate analysis of similarity (ANOSIM) and cluster analysis were used to test for temporal patterns in phytoplankton community composition. The environmental variables that were highly correlated with phytoplankton composition were determined using BEST analysis, a test that compares the ranking within the two matrices of pair-wise similarity coefficients and selects environmental variables "best explaining" community pattern by maximizing a rank correlation between their respective resemblance matrices.38 Principal component analysis (PCA) (based on Euclidian distance) was used to describe overall environmental conditions. The similarity percentage test (SIMPER) was applied to identify environmental variables that are highly correlated with phytoplankton composition shifts. Environmental variables were transformed, if necessary, to meet the criterion of normality, and normalized prior to the comparison. The transforms applied to minimize skewness in the data were log(X+n), where n=0 (DON and PP), n=0.1 (turbidity, wind speed and DIP), n=1 (salinity and PN) or n=-1.5 (discharge).
Daily river discharge monitored at the Fish River gauge did not exhibit strong seasonality (Figure 2A). Discharge maxima occurred in spring 2009 and winter 2010. Overall average discharge (±standard deviation for all the results) during the study period was 3.19±3.26m3s-1. Daily discharge values were averaged for different periods prior to the sampling date (1, 2, 3, 5, 7, 14 and 21days) for statistical analysis. However, all these expressions of discharge were correlated (R>0.6 for periods of less than 7days), so only the 3-day average was used for comparison with phytoplankton community composition. The interval was chosen to allow for the lag between measurement at the gauging station and flushing at the study site and to account for rapid phytoplankton growth rates recorded in the area.20
Depth to the groundwater table was weakly correlated with discharge (R=0.42). The groundwater table was highest during winter and spring 2010 (Figure 2A). Averages of groundwater elevation for the same intervals (1–21days) were highly correlated (R≥0.95), so the 3-day average was used for analysis. Water depth at the sampling site varied from 1.88 to 2.53m, averaging 2.17m, and was not significantly correlated (p>0.05) with discharge nor with groundwater elevation. The salinity at the site was relatively low, 0-16.8, averaging 5.3 (Figure 2B). The relationship between salinity and the 3-day average of discharge was, as expected, negative but the correlation was relatively weak (R=-0.36). Temperature showed strong seasonality, ranging from 10 to 32°C (Figure 2B).

Figure 2 Variation in physical and chemical characteristics at the study site. A) Daily discharge from the Fish River: daily values as well as 3-day average prior sampling, depth to groundwater table at USGS well (the depth to groundwater table is inversely correlated with the volume of GW at the well). C) Variation in dissolved inorganic and organic nitrogen (DIN and DON) and particulate nitrogen (PN). D) Variation in dissolved inorganic and organic phosphorus (DIP and DOP) and particulate phosphorus (PP). E) Variation in chlorophyll a concentration and dissolved oxygen concentration.
Nutrient concentrations were relatively high, with TN and TP ranging from 86-169µM and 1.0 - 5.2µM, respectively (Table 1). Both DIN and DIP were above the limit of detection in all samples: the minima measured were 13.5µM DIN and 0.01 µM DIP. The nitrogen pool was dominated by dissolved species (Figure 2C). Concentrations of DIN and DON were similar, except in late 2008 to early 2009, when DIN was consistently higher, and spring to summer 2010, when DON exceeded DIN. In contrast, the phosphorus pool was dominated by particulate phosphorus (PP) (Figure 2D). Concentrations of DIP were consistently lower than DOP. PN and PP were significantly correlated (R =0.60, p<0.001) although TN and TP were not. The molar PN:PP ratio ranged from 7.5 to 62.6 (mean 17.5±9.9), much lower than the TN:TP ratio (25.5-143, mean 60.8±27.1) (Table 1).
|
Variable |
Min |
Max |
Average |
Chl. p value (R) |
|
Chlorophyll a (µg/L) |
2.2 |
160.5 |
42.3 |
-- |
|
Temperature (C) |
10 |
32 |
22.6 |
0.007 (0.36) |
|
Salinity (ppt) |
0 |
16.8 |
5.3 |
0.253 |
|
Dissolved oxygen (mg/L) |
2.2 |
11.6 |
7.2 |
0.107 |
|
pH |
5 |
9.4 |
7.2 |
0.662 |
|
Turbidity (NTU) |
6 |
60 |
19.8 |
0.433 |
|
NO3(µM) |
9.2 |
67.9 |
40.1 |
0.021 |
|
NO2(µM) |
0.04 |
0.98 |
0.37 |
0.192 |
|
NH4(µM) |
0.7 |
12.5 |
4.8 |
0.257 |
|
PO4(µM) |
0.01 |
0.96 |
0.22 |
0.038 |
|
SiO3(µM) |
30 |
116 |
81.1 |
0.41 |
|
Particulate Nitrogen (µM) |
6.7 |
71.9 |
22.9 |
<0.001 (0.76) |
|
Particulate Phosphorus (µM) |
0.3 |
4.1 |
1.5 |
0.001 (0.43) |
|
Total Nitrogen (µM) |
85.7 |
168.8 |
120.7 |
0.268 |
|
Total Phosphorus (µM) |
1 |
5.2 |
2.3 |
0.021 |
|
DIN:DIP |
17 |
3642 |
365 |
0.399 |
|
DON:DOP |
41 |
2147 |
167 |
0.787 |
|
DIN:DON |
0.26 |
1.97 |
0.93 |
0.024 |
|
PN:PP |
8 |
63 |
18 |
0.001 (0.43) |
|
TN:TP |
26 |
143 |
61 |
0.326 |
|
Discharge (m3/sec) |
1.6 |
17.6 |
3.2 |
0.036 |
|
Groundwater table (m) |
0.7 |
1.6 |
1.2 |
0.231 |
|
Depth (m) |
1.9 |
2.5 |
2.2 |
0.104 |
|
Wind (m/sec) |
0.4 |
2.1 |
0.9 |
0.78 |
|
PAR (mmol/m2) |
39 |
428 |
275 |
0.38 |
Table 1 Minimum, maximum, and average of environmental variables. Discharge and groundwater elevation are averaged for previous 3days, depth, wind and PAR are averaged for previous 24hours. Pearson correlation p values are shown for univariate correlations of Chla values with each environmental variables. The correlation coefficient (Pearson’s R) is shown in parentheses only if the relationship was significant (p<0.01)
The environmental variables were entered into a principal component analysis (PCA). The factors included in the analysis were temperature, salinity, turbidity, and the concentrations of DIN, DON, PN, DIP, DOP, PP and silicate. Discharge and ground water elevation were included as mean of previous 3days and wind speed, depth at the site and PAR were expressed as the mean for the previous day. The first four PCs met the Kaiser criterion39 of eigen value exceeding one and explained 70.5% of the total variation (Table 2). There was a distinct seasonal pattern in the distribution of factor scores for PC1 and PC2, with samples collected in the summers and winters forming the outer bounds of the distribution and samples from springs and falls lying in between them (Figure 3). There was also inter-annual variation within the summer and winter samples. There were significant differences in PC1 scores between winter 1 and 2 and summer 1 and 2 (Tukey’s post-hoc test following 1-way ANOVA, 0.01< p<0.0001). Differences were less marked on PC2: there were no significant differences between winters 1 and 2 nor between summers 1 and 2 but were between winter 1 and each summer and between winter 2 and summer 2 (0.001<p< 0.0001). There were no significant differences (p>0.05) between PC1 or PC2 scores in the two springs and in the two falls.
|
PC |
1 |
2 |
3 |
4 |
|
Eigenvalues |
4.24 |
2.68 |
2.21 |
1.44 |
|
% Variation |
28.2 |
17.9 |
14.7 |
9.6 |
|
Cum. % Variation |
28.2 |
46.1 |
60.8 |
70.5 |
|
Variable |
PC1 |
PC2 |
PC3 |
PC4 |
|
Temperature |
-0.307 |
-0.374 |
0.112 |
-0.195 |
|
Salinity |
-0.268 |
0.25 |
-0.184 |
-0.147 |
|
Depth |
-0.216 |
-0.366 |
-0.086 |
-0.367 |
|
Turbidity |
0.238 |
-0.329 |
-0.183 |
0.066 |
|
Wind Speed |
0.242 |
-0.075 |
-0.289 |
-0.235 |
|
PAR |
-0.293 |
-0.322 |
0.142 |
0.040 |
|
GW Table |
0.210 |
0.284 |
-0.379 |
-0.222 |
|
Discharge |
0.400 |
-0.086 |
0.066 |
0.004 |
|
DIN |
0.041 |
0.456 |
0.244 |
-0.047 |
|
DON |
0.252 |
-0.196 |
0.394 |
0.252 |
|
PN |
-0.176 |
-0.207 |
-0.269 |
0.549 |
|
DIP |
0.378 |
0.097 |
-0.187 |
0.049 |
|
DOP |
0.208 |
-0.23 |
-0.194 |
-0.315 |
|
PP |
-0.065 |
0.000 |
-0.509 |
0.428 |
|
SiO3 |
-0.308 |
0.084 |
0.204 |
0.208 |
Table 2 Eigenvalues of the first 4 principal components in a Principal Components Analysis and the associated Eigenvectors for the input variables. Eigenvectors in bold are significantly correlated with the PC
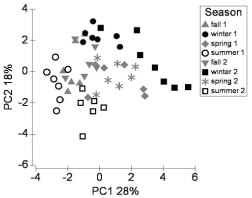
Figure 3 Biplot of the first two principal components in the PCA of environmental factors, showing the sample PC scores. Sample scores are classified by year and season. Seasons were defined in terms of trimesters: winter: Dec, Jan, Feb; spring: Mar, April, May; summer: Jun, July, Aug; fall: Sep, Oct, Nov.
Significant correlations between the input variables and principal components (PCs) were determined using the broken-stick method.40 PC1, which explained 28.2% of the variability was significantly correlated with 12 variables (Table 2). It was the only PC that was significantly correlated with discharge, DIP and silicate. Differences in discharge and its closest correlates (DIP, R=0.69; silicate, R=-0.54) were largely responsible for the inter-annual separation of samples in the winters and summers (Figure 3) (Figure 2). PC2 was significantly correlated with 9 variables (Table 2). In contrast to the parameters that were correlated only with PC1 (discharge, DIP and silicate), each of the correlates of PC2 except PN and DOP varied significantly between seasons (0.01< p< 0.0001, ANOVA), contributing to the seasonal separation on both PC1 and PC2. The only parameter that was not significantly correlated with PC1 or PC2 was PP, which was correlated with PCs 3 and 4 (Table 2). Winter was the season with the highest dynamic range in discharge (Figure 2).
There was a strong seasonal pattern in DO (mgL-1), with the expected minima at high temperatures in the summer (Figure 2E). There was no significant (p<0.05) relationship between Chla and DO. There was a weak correlation (R =0.30, p=0.01) if Chla was offset by one sample period (i.e. when DO was compared to Chla collected two weeks previously). The relationship was not significant when Chla was lagged by 4 weeks. The best predictor of DO was temperature (R=-0.72, p<0.001). Inclusion of Chla, TN and TP to the regression did not improve the fit.
In contrast, there was no seasonal trend in Chla (Figure 2E). The mean concentration was 42.3±3.1µgL-1 and the three highest values (160±6.5, 111±5.0 and 108±1.3µgL-1) occurred in spring and fall 2009 and summer 2010. Pearson correlations between Chla and other variables were significant only for temperature and particulate nutrients (Table 1). Particulate nutrients and Chla are expected to be correlated since they both reflect the presence of cells/particulates, so this relationship is not causative. Temperature/seasonality was only marginally significant. In addition to Pearson correlations, data were pretreated and analyzed using a BEST analysis38 based on Euclidean distance. The highest correlation was with a combination of DIP, DIN, discharge, temperature, and TP (Spearman Rho=0.43, p=0.01). DIP and DIN combined accounted for much of the correlation (Rho=0.37) and were included in all optimal combinations of 2-5 variables. The single factor with highest correlation was DIP (Rho=0.28). This suggests that phytoplankton abundance as Chla was more likely to be limited by phosphorus than nitrogen during the study, as would be predicted from the high molar DIN:DIP ratios of 17–3,642 and also high significance of DIP in explaining PC1. There was no statistically significant (p>0.05) relationship between Chla and zooplankton abundance in either tests.
Phytoplankton composition
Microscopic counts of phytoplankton were aggregated to the class level for all analyses. Cyanobacterial numbers were recorded consistently but because pico-cyanobacteria (that are likely to have dominated the class41,42) are not amenable to identification by light microscopy, the overall cyanobacteria counts were most likely underestimated. However, there was a statistically significant (R=0.75, p<0.001) relationship between concentrations of zeaxanthin and the microscopic counts. Zeaxanthin is present in Rhodophyceae and in the Raphidophyceae, however the former were not found at the site and numbers of the latter were low in most samples, so zeaxanthin was treated as taxonomic marker pigment for cyanobacteria. Murrell and Lores41 reported a regression coefficient for calculating cyanobacteria number based on zeaxanthin concentration. Based on this regression coefficient, calculatedpico-cyanobacterial abundance would be 2.1±2.0x109 cellsL-1, similar to the numbers reported by Murrell and Caffrey42 from Weeks Bay (2.4±1.9x109 cellsL-1) and about 3 orders of magnitude higher than the counts reported here (3.5± 3.4x106 cellsL-1). This suggests that microscopic counts in this study underestimated the actual abundance of cyanobacteria. However, the strong significant relationship between cyanobacteria abundance and zeaxanthin suggests that even though the cyanobacterial counts were not accurate, they were sufficiently precise to be representative of temporal variation trends.
The phytoplankton community composition (based on cell counts) was analyzed using cluster analysis and ordinated by multi-dimensional scaling (MDS). All of the taxonomic classes were included in the analysis (diatoms, dinoflagellates, chlorophytes, cryptophytes, raphidophytes, cyanobacteria, prasinophytes, euglenophytes, chrysophytes, dictyophytes, prymnesiophytes). The analysis generated eight clusters (A-H) at the 50% similarity level (Figure 4). Three of these (A, E and H) contained either one or two samples. Cluster A was dominated (90%) by cyanobacteria; Cluster E was dominated by cyanobacteria (68%) and cryptophytes (28%); and Cluster H was a bloom of raphidophytes (89%). The highest observed Chla concentration (160µgL-1, in March 2009) was in the raphidophyte bloom, which contained 9x109 cells L-1 of Heterosigma spp.
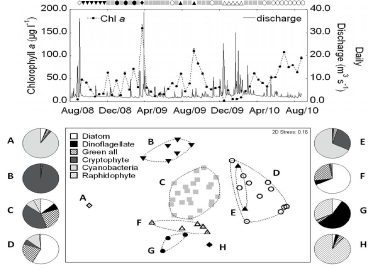
Figure 4 Upper panel: Variation in chlorophyll a concentration and discharge. Symbols above the upper panel correspond to the clusters defined by multi-dimensional scaling (MDS) analysis based on the phytoplankton composition (lower panel). The symbols above the upper panel therefore represent phytoplankton composition at the specific time, chlorophyll a concentration and discharge. Lower panel: Multi-dimensional scaling plot of class-level community composition data. Symbols correspond to clusters (A-H) defined at 50% similarity (see text for details). Note that Cluster E is distinct from Cluster D: the coincidence is an artifact of ordination in only 2 dimensions. The pie charts show the average phytoplankton abundance for samples from each cluster. For clarity all chlorophycean lineages (chlorophytes, prasinophytes and euglenophytes) have been grouped as “Green all” and classes with minor contributions (chrysophytes, dictyophytes, and prymnesiophytes) have been omitted from the pie charts.
Of the clusters with 3 or more samples, Cluster C had the highest diversity. The most numerous taxon in Cluster C, which was observed in all seasons, was the cryptophytes (40%). The second major Chla peak (111µgL-1 in September 2009) was in Cluster C and contained 12x106 cellsL-1 of chlorophytes. There were two other clusters, F and G, found at low temperatures (Figure 5). As with the environmental data (Figure 3), these were separated by year. Cluster G, which occurred in winter 2008 - 2009, had the highest proportion of dinoflagellates (55%) of all the clusters, with a high contribution from raphidophytes (32%). The dominant dinoflagellates were Prorocentrum minimum, Heterocapsa triquetra and Akashiwo sanguinea. Cluster F, which occurred in winter 2009 - 2010, was dominated by diatoms (68%) and had a higher proportion of chlorophytes (24%) than any other cluster. The remaining two clusters, B and D, were observed at medium to high temperatures in fall and (with one exception) in late spring/summer, respectively (Figure 4). Cluster B was dominated by cryptophytes (99%). Cluster D was dominated by diatoms (58%) and chlorophytes (23%). The third major Chla peak (108µgL-1, in June 2010) was in Cluster D and contained 30x106cellsL-1 of diatoms. Although both Clusters D and F were dominated by diatoms, there were differences in their composition. The commonest species in the spring/summer assemblage in Cluster D was Synedropsis karsteteri, while the commonest species in the winter assemblage in Cluster F were Cyclotella spp, Skeletonema spp, Navicula spp, and Nitzschia spp.

Figure 5 Upper panel: Variation in temperature and phytoplankton composition. Symbols in the upper panel correspond to the clusters defined by MDS for community composition (Figure 4). The position of the symbols in the upper panel corresponds to temperature at the time of sampling. The three ranges are low T (10.0–18°C), mid T (18–25°C) and high T (25–32.0°C). The x-axis is the same for both panels.
Zooplankton abundance ranged from 2, 820L-1 (9/23/2008) to 452, 000L-1(4/6/2010), averaging 78,800±88,900 cellsL-1. Based on total number of cells (sum of phytoplankton and zooplankton counts) zooplankton number comprised on average 1.9±3% of the total number of cells, with the highest contribution of 19% on 1/27/09.
Environmental correlates of community composition
The environmental correlates of phytoplankton community composition were tested by BEST analysis. The highest correlation was with a combination of temperature, salinity, silicate, TP and DIN (Spearman Rho=0.400, p=0.01). The single factor with the highest predictive power was temperature (Rho=0.245).Because temperature was such a dominant driver of the community dynamics, it masked other, less prominent, drivers. The community composition clusters were therefore further subdivided based on temperature and new temperature-independent comparisons were calculated within these subdivisions. This way any temperature effect was removed which allowed for detailed evaluation of remaining environmental conditions. The comparison was made between the clusters that occurred at low temperatures (10-18°C; Clusters C, F and G) and between the clusters that occurred at high temperatures (25- 32°C; Clusters B, C and D) (Figure 5). These two sets of clusters correspond to the ‘winter’ and ‘summer’ populations (Figure 3), which differed between years in PC1 scores, PC1 being loaded heavily on the hydrological data. Clusters with only one or two samples (A, E and H) were omitted from the analysis. Clusters at low temperature and high temperature were compared using ANOSIM and SIMPER. Components of the nitrogen and phosphorus pools were grouped as TN and TP because of the potential for rapid turnover in inorganic vs organic species when compared to the time associated for taxonomic succession. The exception was the DON:DIN ratio that was included because of its potential as an index of succession.43 There was a significant difference in the environmental variables based on phytoplankton clusters (one-way ANOSIM R=0.336, p=0.001).
In the low temperature subdivision, variables that contributed the most to the dissimilarities between clusters C, F and G were discharge (24%) and zooplankton (21%), turbidity (14%). Of the other variables, TP, salinity and silicate also contributed but less than 14% in aggregate (SIMPER analysis). Discharge and turbidity were consistently low when the dinoflagellate- and raphidophyte-dominated Cluster G was present and were higher and more variable when the diatom-dominated Cluster F was present (Figure 6). Zooplankton abundance was much higher when Cluster G was present than when Cluster F was. The conditions when Cluster C, the mixed assemblage, was present were intermediate between those characteristic of Clusters F and G.
In the high temperature subdivision, clusters B,C,D differed predominantly due to salinity (18%) and groundwater (14%); less prominent effects were attributed to depth, PAR, silicate, DIN:DON and TN (<14%) (SIMPER analysis).This is consistent with the differences in both PC1 and PC2 scores between the summer populations (Figure 3) (Table 2). Salinity was higher during the presence of the cryptophyte-dominated Cluster B than when the other two clusters were present and the groundwater elevation was higher when the diatom-dominated Cluster D was present than when the other two clusters were (Figure 6). Of the environmental factors with the less prominent effects: the water was deeper and PAR was consistently higher when the cryptophyte-dominated Cluster B was present than when the mixed assemblage Cluster C was present.
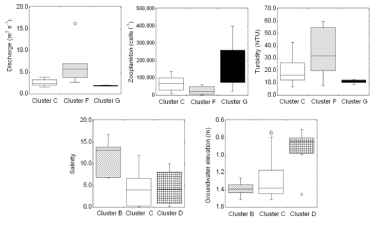
Figure 6 Box plots illustrating the distribution of environmental parameters that contribute most to differences in conditions between the low-temperature clusters (C, F and G) and high-temperature clusters (B, C and D). The parameters shown are ranked in the upper 3 contributions to dissimilarity in two pair-wise comparisons, based on SIMPER.
Although the analysis can not extend to the clusters containing 1 or 2 samples, it is likely that discharge was responsible for the transition between the cyanobacteria-dominated Cluster A and the cryptophyte-dominated Cluster B: there was a major discharge event in the interval between the two samples (Figure 4). Similarly, there was a minor discharge event before the raphidophyte bloom (Cluster H) and a major one after it (Figure 4).
Ecosystem state was inferred from three areas of interest: nutrient pool sizes, Chla and phytoplankton community composition. The former are routinely monitored in water-quality assessments but we argue that it is critical to evaluate three factors to assess ecosystem state accurately. Prior work in the system has shown that short-term variability in Chla and water clarity can be comparable to seasonal variability,44 as has been found in similar shallow-water systems elsewhere.45,46 The biweekly sampling regime used in this study could not resolve such variability but even so, there were strong patterns in community structure that were driven by the interaction of seasonality and hydrology. Temperature is a common correlate of community structure10,47 and the hydrology can influence both nutrient availability and water clarity. These data show that differences in hydrology within a given temperature regime can translate to a community that is benign in terms of the component species or one that can develop into a harmful algal bloom.
Many studies of harmful algal blooms have focused on the role of nutrient loading.3,48,49 TN and TP were very high in Weeks Bay (up to 169 and 5.2µM) compared to concentrations in other systems of similar size,18,50 but within the ranges reported by previous studies at the same study site44,51and low compared to many systems with high human population density in the watershed.52 Increased discharge was correlated with increased DIP and decreased silicate and, for winter samples only, decreased DIN. Correlations between high discharge, decreased nitrate and increased phosphate concentrations in the Fish River have also been shown in decadal data series.23,24 A reduction in DIN and an increase in TP with discharge events have also been documented previously in the same drainage basin by Lehrter20 who attributed them to the interaction of persistent groundwater inputs and episodic surface flow. Nitrate concentrations in the groundwater are very high (up to 2mM) and exceed regulatory limits25 but phosphorus is likely to be sediment-bound.53 The molar TN:TP ratio in samples from the aquifer exceed 240:1 (unpublished data). The persistent groundwater inputs therefore result in high N:P, with the associated likelihood of P limitation.54 Lehrter20 argued that episodic precipitation events resulted in high discharge, driven by surface runoff that diluted the nitrate-rich groundwater and enriched phosphorus through transport of particle-bound phosphate. Our data are not fully consistent with this scenario, although the difference between a flux of surface runoff rather than increased submarine groundwater discharge would explain the differences in discharge-nutrient relationships between Weeks Bay and groundwater-driven Little Lagoon,55 another shallow-water system about 20km south of Weeks Bay.
In this study, discharge events were correlated with reduced DIN, but only during the winter. They were also correlated with reduced silicate, which is high in groundwater because of the dominance of siliceous sediments in the aquifer.22 However, there was no correlation between either particulate phosphorus, which dominates total phosphate, or total phosphorus and discharge, although there was between DIP and discharge. There are three mechanisms, which are not mutually exclusive, that might account for this. The first is that the runoff supplied DIP directly. The second is that the DIP was derived by desorption from particulate material. Although discharge, turbidity and DIP were correlated with each other, none was correlated with particulate phosphorus, which dominated the phosphorus pool at all times. If this is the mechanism, there must be a decoupling of the dissolved and particulate pools. A third possibility is that DIP is released from the sediments. High discharge can result in resuspension, altering nutrient fluxes as DIP is released from the sediments during resuspension and it is replaced by phosphate from deeper sediments.21,56,57 The fact that discharge, turbidity and DIP were correlated with each other, although none was correlated with particulate phosphorus, is most consistent with this scenario.
Chlorophyll a concentrations alone are not a good indicator of ecosystem health. We documented three events when chlorophyll a concentration exceeded 100µgL-1 (Figure 4). The phytoplankton community composition was very different during these high-Chla events: two were dominated by harmless, non-toxic chlorophytes and diatoms, but the third was dominated by the potentially toxic raphidophytes Heterosigma spp. Again, monitoring chlorophyll a concentration is a common practice among the state agencies yet it does not accurately reflect the ecosystem conditions. Phytoplankton species composition can trigger a variety of ecosystem responses. Despites its simplicity and convenience, ecosystem status cannot be determined by monitoring chlorophyll a concentrations alone.
The environmental factors that were most highly correlated with Chla were DIP and DIN, and secondarily, temperature, discharge, and TP. These results are consistent with those of Lehrter51 who found that discharge/mixing time, TP and PAR are the best descriptors of Chla in Mobile Bay. The correlations with a nutrient and a proxy of residence time and the lack of a correlation with zooplankton abundance (a proxy for grazing) are consistent with a system dominated by transport rather than the balance between growth and grazing.58 Even though PN:PP ratios fell within reported ranges of the Critical Ratio, the transition point between N- and P-limitation of c. 20–40mol mol59–61, we concluded that phosphorus, not nitrogen, is more likely to be a limiting nutrient. The evidence supporting our hypothesis is based on: 1) high TN:TP and DIN: DIP ratios that were always in excess of the Redfield Ratio; 2) DIP in all samples that was at or below the reported range of km for phosphate uptake,62 and 3) DIP as the factor with the highest explanatory power forvariation in Chla.
Eutrophication and high phytoplankton biomass frequently lead to hypoxia. For example, Greene et al.63 predicted summer hypoxia in the northern Gulf of Mexico based on nitrate, TP and discharge in winter and spring. Although our study site had variable but overall high nutrient loads and high Chla(42.3±31µgL-1)throughout the year, low DO levels were restricted to summer and correlated with increased temperature. A total of 6% of the biweekly DO values (taken at 13:00) and 12% of the continuous readings from the mooring (day and night) were below 4mgL-1. Even though the site was very shallow (~2m), stratification occurred. Several fish kills (menhaden) were reported in adjacent areas as a result of hypoxia during the study (E. Brunden, Weeks Bay NERR, personal communication). Although elevated nutrient concentrations are a common cause for hypoxic/anoxic conditions in coastal areas, we found no evidence for a simple causal relationship. In this study, temperature had the highest explanatory power in explaining hypoxic conditions and inclusion of Chla and/or nutrients did not increase the amount of variability explained. Where nutrient loads are very high but unassimilated, the nutrient threshold needed to support a phytoplankton bloom can be exceeded while the bloom dynamics are controlled by other factors. In this study, it appears that the accumulation of biomass was driven as much by the discharge dynamics (flushing vs. retention) as by nutrient concentration.
The phytoplankton composition was very dynamic, oscillating between high abundances of cryptophytes, chlorophytes or cyanobacteria, interspersed with high densities of the raphidophytes Heterosigma spp., the dinoflagellates Prorocentrum minimum and Heterocapsa triquetra, and the diatoms Synedropsis karsteteri and Cyclotella spp. Temperature was the single variable that explained the most variation in the phytoplankton community composition overall, as seen in other studies.10,47 The study site was characterized by both seasonal and inter-annual variability in phytoplankton composition. High abundances of the dinoflagellate Prorocentrum minimum occurred only in colder months. Cyanobacteria reached their highest densities in warm months. Cyanobacteria can comprise a significant fraction of the Chla. Murrell and Caffrey42 documented that cyanobacteria contributed 30-50% of total Chla, but may reach up to 100% in summer in Weeks Bay. Cyanobacteria dominated the assemblage from Mobile Bay in the summer, based on marker pigments64 and their dominance over eukaryotes at high temperatures has been attributed to higher growth optima for temperature.65,66 Diatoms as a class were abundant over the entire range of temperature, although Cluster D, which was dominated by Synedropsis karsteteri, was restricted to warm periods, as reported previously by Prasad and Livingston67. In contrast, Skeletonema spp., Cyclotella spp. and Nitzschia spp. were present throughout the year.
Phytoplankton dynamics and production are controlled by physical, chemical and biological factors.11 We have shown that river discharge, groundwater elevation, salinity, TP and zooplankton abundance were all correlated with shifts in phytoplankton composition within the same temperature range (Figure 6). The effect of river discharge on phytoplankton composition likely occurs in two steps. Step one includes all the physical processes associated with river flow. High river discharge flushes most of the phytoplankton cells present at the site. These cells are replaced by cells that originated from one or more sources: 1) freshwater phytoplankton arriving to the site with the discharge; 2) phytoplankton originally present at the site that could reproduce more rapidly than the losses due to flushing; 3) estuarine phytoplankton advected to the site with tides and winds; and 4) benthic microalgae that were introduced to the water column via resuspension associated with the discharge event. The second of these is associated with the environmental conditions created by discharge that will consequently affect phytoplankton growth. The growth rate of the newly established population will depend on their responses to nutrient availability, temperature, and light, and the grazers, competitors and viruses present. River discharge directly or indirectly affects all of these factors except temperature. The production and loss of phytoplankton biomass are co-occurring processes and there is a dynamic balance in between them.8
We found relatively high dinoflagellate abundances during a low-discharge winter (Cluster G) while diatoms dominated during a winter that was characterized by high and variable discharge (Cluster F). The frequency of high discharge events will determine the residence time and level of disturbance. Low discharge events, which are characterized by reduced turbulence and high residence times, benefit slow-growing classes of algae, specifically dinoflagellates.62,68,69 Long residence time allows for accumulation of phytoplankton where growth rates exceed loss rates.58 High disturbance can negatively impact dinoflagellates via physical damage and behavioral modification.62 In contrast, diatoms, and particularly estuarine diatoms, are well equipped for high turbulence and the resulting variable light conditions70 and are often more tolerant of environments characterized by strong mixing than dinoflagellates.62,68 Even though high turbulence may break up diatom chains it prevents diatoms from sinking and can stimulate their growth.
Grazing rates were not quantified in this study, but zooplankton abundance was. Although zooplankton abundance had no explanatory power for Chla concentrations, it was correlated with phytoplankton composition, specifically during the low-temperature conditions associated with the dinoflagellate-dominated Cluster G rather than the mixed population Cluster C and the diatom-dominated Cluster D. Previous research in Mobile Bay estimated the mean annual microzooplankton grazing rate at 0.57-1.10d-1 and argued for an effect on phytoplankton through direct consumption but also via excretion of nitrogen.71 Cluster G, which was dominated by dinoflagellates and raphidophytes, was associated with high zooplankton abundance. Both taxa are frequently chemically-defended and are likely subject to low grazing pressure, due to their potential toxicity and palatability. For example, Akashiwo sanguinea, which was found at the site, was reported to avoid grazing by microzooplankton.72 One the other hand, another dominant species, Prorocentrum minimum, can be grazed freely by ciliates and copepods.73–75 Whether the association between high zooplankton numbers and the dinoflagellate-dominated assemblage is a result of selective grazing or a stimulatory effect of regenerated source of nutrients cannot be determined on the basis of correlation.
While there is an abundant literature associated with the conditions likely to favor dinoflagellates vs diatoms, which is consistent with the differences in environmental conditions between the dinoflagellate-dominated and diatom-dominated winter assemblages documented here, there is no such literature for differences between estuarine cryptophytes and diatoms. The two environmental factors that separated the mixed assemblages Cluster C, cryptophyte-dominated Cluster B and the diatom-dominated Cluster F were salinity and the height of the water table. The cryptophytes dominated when salinities were relatively high and the diatoms dominated when the depth at the site was relatively high. Depth can be important in providing a refuge from grazers or photo inhibition. Discharge was low during the periods when these high-temperature assemblages were present: <3.5m3s-1 in all but one case. All of the indices that separate the three clusters except silicate are correlated with the depth of the water table and silicate increases in the presence of groundwater discharge. Consequently, it is likely that the driver for shifts in community composition between the high-temperature clusters is submarine groundwater discharge.
The role of hydrology and flushing in regulating phytoplankton abundance is well established.58 We argue that it has an equally important role in structuring the phytoplankton communities in shallow-water environments, with the potential for favoring assemblages dominated by bloom-forming dinoflagellates under conditions of low discharge (this study) as well as communities dominated by blooms of potentially-toxic diatoms in the genus Pseudo-nitzschia.64,76 The dominance of these groups under low- and high-discharge regimes reflects habitat stability as much as habitat richness. In the nascent stages, neither type of bloom will be detected by monitoring programs that rely on Chla and nutrient concentrations alone. However, monitoring programs do provide the inputs needed for predictive models of bloom formation. For these reasons, we recommend an increased emphasis on monitoring community structure, even though the costs are high.
This study describes phytoplankton abundance, community composition, and water quality in a shallow estuary (Weeks Bay, Alabama (USA)) over period of 2 years. We demonstrate that Chlorophyll a concentrations alone are not a sufficient descriptor of ecosystem health. We documented three events when chlorophyll a concentration exceeded 100µgL-1, however the phytoplankton community composition was very different during these events: two were dominated by harmless, non-toxic chlorophytes and diatoms, but the third was dominated by the potentially toxic raphidophyte. It is both phytoplankton quantity and quality that trigger negative ecosystem responses and both should be regularly monitored. Phytoplankton production and composition shifts are controlled by physical, chemical and biological factors. Shallow estuary is a very dynamic environment, connecting riverine discharge, salt water intrusion, benthic fluxes and biological processes. In this study, it appears that the accumulation of biomass was driven as much by the discharge dynamics (flushing vs. retention) as by nutrient concentration. Temperature was the single variable that explained the most variation in the phytoplankton community composition.
L. Novoveská was supported by a NOAA NERR Graduate Fellowship (NA07NOS4200023). This research was supported by Dauphin Island Sea Lab. We particularly thank to Laura Linn, Emily Goldman and Justin Liefer for assistance in sample analysis. We thank the dedicated staff of Weeks Bay National Estuarine Research Reserve, particularly Eric Brunden, Dr. Scott Phipps, and Sarah Johnston for support throughout the project; and Bill Smith and Carol Dorsey of Alabama Department of Public Health for their generous assistance with phytoplankton identification.
The author declares that there are no conflicts of interest in relation to this article.

©2019 Novoveská, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.