Journal of
eISSN: 2378-3184


Research Article Volume 10 Issue 2
Ministry of Jihad -e- Agriculture, Agricultural Iranian Fisheries Science Research Institute (IFSRI), Caspian Sea Ecology Research Center (CSERC), Iran
Correspondence: SM Vahid Farabi, Department of Aquaculture, CSERC, IFSRI, Iran
Received: March 08, 2021 | Published: March 26, 2021
Citation: Farabi SMV, Bahmani M, Darzi MG. Study of the osmotic regulation processing in the juvenile Persian sturgeon, Acipenser persicus. Borodin, 1897 in transfer directly to the Caspian seawater. J Aquac Mar Biol. 2021;10(2):60-64. DOI: 10.15406/jamb.2021.10.00307
The aim of this study was to transfer directly juvenile Acipenser persicus from freshwater to the Caspian Seawater (CSW). The fish in different age and size (weight (W) & length (L)) groups (I: 35days, W: 2.75±0.15g & L: 7.93±0.47cm; II: 50days, W: 3.15±0.12g & L: 8.31±0.28cm; III: 65days, W: 4.33±0.12g & L: 9.39±0.29cm; n=90 for each group) and in two different saline water (FW: <0.5‰ and CSW: 12.5‰) were investigated. The fish were transferred directly from FW to CSW. After 168 hours, blood samples were taken from the fish. The results showed that the survival in all groups in CSW was from 56% up to 76% and increased with increasing age and weight of fish. Some hematological parameters and levels of cortisol, osmolarity and ion concentration (Na+, K+, Ca+2 and Mg+2) in the plasma were determined. The functional levels of the mechanism of osmotic and ionic homeostasis were similar in all groups (P>0.05) but differed in experimental media (P<0.05). Significant differences were observed between the levels of plasma ion concentrations in different media (P<0.05). Plasma Na+, K+ and Ca+2 concentrations were higher than those of FW media, but lower than in Brackish water media (P<0.05). Plasma Mg+2 concentrations were lower than those of FW and Brackish water media (P<0.05). The Hct, Hb, MCV, MCH and plasma osmolarity decreased and MCHC and plasma cortisol levels increased with increasing salinity (P<0.05). But the number of WBC and RBC did not change (P<0.05) and reached a stable state. Therefore, it is possible to release juvenile sturgeon directly from FW to CSW with increasing weight and age.
Keywords: acipenser pesicus, hematological parameters, ion, cortisol, osmolarity
In the current situation, it is necessary to restore the sturgeon stocks of the Caspian Sea. Because sturgeon fishing has declined sharply over a 110-year period leading up to 2006, from 29.8 to 0.38 thousand tonnes.1 The declining trend of fishing and exploitation of sturgeon in the Caspian Sea continues continuously. Because the existing threats, including river pollution, overfishing continue.2
On the other hand, one of the main problems in fish stock regeneration activities is the loss of juvenile fish due to artificial reproduction during release into the natural environment. Most juvenile deaths occur when entering the wild.3,4,5 Studies by Sanchez et al.6 showed that one of the important indicators of success or failure in the process of tolerance to environmental salinity and ion-osmotic regulation is the survival rate of fish according to age and size6. Various scientists around the world have studied the ionic and osmotic regulation of fish. In these studies, hematological, serum and hormonal parameters along with measurement of serum ions and plasma osmolarity were generally examined. Numerous studies have also been performed on sturgeon, for example: in Huso huso, A. persicus, A. gueldenstaedti, A. stellatus, A. nudiventris from Caspian Sea7–12 in A. transmontanus,13 in A. naccari14,6, in A. bravirostrum and A. oxyrhynchus.15,16 The aim of the present study was to investigate the osmotic and ionic regulation of A. persicus juveniles from artificial reproduction of sturgeon propagation centers for direct release into the Caspian Sea.
In the direct transfer of fish from fresh water to seawater, some hematological, serum, hormonal and ion-osmotic indices in Acipenser persicus, of different groups (I: 35days, BW: 2.75±0.15g, TL: 7.93±0.47cm; II: 50days, BW: 3.15±0.12g, TL: 8.31±0.28cm; III: 65days, BW: 4.33±0.12g, TL: 9.39±0.29cm) in freshwater (FW :< 0.5‰) and the Caspian Seawater (CSW: 12.5‰) were investigated (Figure 1).
Figure 1 Location of studies in the Caspian Sea Ecology Research Center in the southern region of the Caspian Sea.
Each group consisted of 90 fish in three experimental units (n=30). The fishes were directly transferred from FW to CSW. The possible repercussions of ion-osmoregulatory processes on some classical indicators were examined at the end of 168 hours' fish salinity tolerance. Blood cell count (Red blood cells: RBC, white blood cells: WBC), blood parameters (hemoglobin concentration: Hb, hematocrit: Hct) were measured by microscope and haemocytometers (standard Neubauer cell counting chamber) over cells suspended in Rees-Escher’s solution. The Hct were measured at 16329.6 g for 5min in a clinical centrifuge (Hettich-D7200 Tuttlingen: Germany). The hemoglobin concentration (Hb) was determined using the cyanmethemoglobin method with spectrophotometry (CECIL- CE1020: Germany) at 540nm. Some blood parameters were calculated by the following equations.18,19
Mean cell volume (MCV) =Hct*10*RBC־¹
Mean cell hemoglobin concentration (MCHC) =Hb*100*Hct־1
Amount of hemoglobin per erythrocyte (MCH) =Hb*10*RBC־¹
The plasma samples were extracted from fish blood by centrifugation (Hettich-D7200 Tuttlingen: Germany) at 453.6g for 5min and then stored at -20°C. After plasma defrosting, ion concentrations (Na+, K+: by flame photometer Corning 405C: IRI; Ca²+and Mg²+: by with an absorption spectrophotometer UNICO 3115233: USA), cortisol (with competitive enzyme immunoassay and automatic instrument for ELISA kits on microplate), and osmolarity (cryoscopy method by (Roebling Nr.9610003. Type 13: Germany) were measured18,19 (Figure 2).
Three age groups (35, 50 and 65 days) were compared to determine the survival rate and salinity stress tolerance. Fresh water was the control group. The results showed that the experimental groups had more than 56% survival after 168 hours. Therefore, mortality was not observed higher than 44% in all groups in CSW. Survival rate was increased with increasing fish age in CSW (Figure 3).
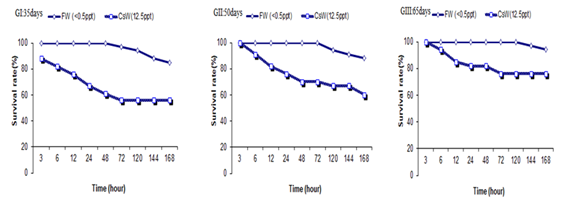
Figure 3 The survival rate of Persian sturgeon in 35, 50, and 65-day ages, in transfer directly from freshwater to the Caspian Seawater.
The results of counting blood cells and measuring and calculating blood indices, cortisol levels and plasma osmolarity are shown in Table 1. Also, the concentration values of blood plasma ions and environmental water of fish are shown in Table 2.
|
Juvenile's group: n=10 |
Hct |
RBC |
WBC |
Hb |
MCV |
MCH |
MCHC |
Cortisol |
Osmolarity |
|
% |
103cellsµl-1 |
103cellsµl-1 |
g/dl |
fl |
pg |
% |
ng/ml |
mOsml/l |
|
|
In FW: <0.5 ‰ and Osmolarity: 5±2 mOsml/l |
|||||||||
|
I .35days |
38.80±2.86 |
679.02±75.93 |
12.90±2.54 |
5.10±0.53 |
565.52±27.28b |
74.08±1.31c |
13.12±0.59b |
18.74±0.92 |
241.50±3.34 |
|
II.50days |
38.80±3.01 |
679.21±71.71 |
13.45±2.31 |
5.10±0.56 |
573.36±26.38b |
75.11±2.71bc |
13.12±0.73b |
18.87±0.50 |
243.04±3.13 |
|
III .65days |
38.80±2.03 |
639.39±80.20 |
14.53±1.43 |
5.36±0.39 |
611.48±40.04a |
84.42±5.74a |
13.81±0.49a |
18.81±0.62 |
240.60±4.22 |
|
CSW: 12.5 ‰ and Osmolarity: 407±2.92 mOsml/l |
|||||||||
|
I .35days |
21.80±1.87 |
668.12±69.25a |
13.50±1.50 |
5.18±0.57 |
327.10±15.41b |
77.63±5.43b |
23.72±0.92 ab |
31.24±1.29 |
297.30±4.79 |
|
II.50days |
21.90±2.18 |
662.32±19.32b |
13.05±1.70 |
5.28±0.62 |
351.48±25.27 a |
84.69±7.62a |
24.08±1.15a |
31.66±0.70 |
296.10±4.15 |
|
III .65days |
21.80±2.2 |
623.21±34.66 ab |
13.15±0.82 |
5.02±0.72 |
343.75±19.46 ab |
79.04±8.06ab |
22.95±1.35b |
31.82±0.89 |
295.00±5.73 |
Table 1 Hematological parameters, concentration of cortisol and osmolarity in blood plasma of juveniles' Acipenser persicus after acclimation for 168 hours to different media
Note: The Values with different superscript and subscript letters are significantly difference in age and size classes of different in each experimental media within each column (Means SD and p<0.05)
|
Media |
In Fresh Water: <0.5 ‰ |
In Caspian Sea Water:12.5 ‰ |
||||||
|
Juvenile's group: n=10 |
Ion Concentration mEq/l |
Ion Concentration mEq/l |
||||||
|
Na+ |
K+ |
Ca²+ |
Mg²+ |
Na+ |
K+ |
Ca²+ |
Mg²+ |
|
|
I .35days |
124.90±1.37a |
1.81±0.05a |
4.84±0.09a |
0.62±0.02 b |
151.32±1.16b |
2.03±0.05b |
5.56±0.09b |
1.75±0.05b |
|
II.50days |
125.10±2.02a |
1.79±0.06a |
4.86±0.09a |
0.65±0.13b |
151.33±2.21b |
2.02±0.04b |
5.57±0.07b |
1.77±0.09b |
|
III .65days |
124.13±3.11a |
1.83±0.06a |
4.88±0.01a |
0.63±0.06b |
152.50±2.88b |
2.03±0.05b |
5.54±0.05b |
1.74±0.06b |
|
Water: n=5 |
28.84±2.86b |
0.39±0.03b |
2.04±0.15b |
1.11±0.16a |
175.88±4.56a |
2.41±0.35a |
20.46±0.82a |
63.3±3.06a |
Table 2 Ion concentration of blood plasma in juveniles' Acipenser persicus and environment water after fish acclimation to different salinity for 168 hours. (Means±SD)
Note: The values with different letter (between groups and water) within each column have shown significantly difference (p<0.01)
The osmotic and ionic regulation were shown in all groups and differed in experimental waters (P<0.05). Significant differences were observed between the levels of plasma ion concentrations in different waters (P<0.05). The plasma level of Na+, K+, and Ca+2 ions were higher than those of FW but lower than in CsW (P<0.05). The plasma level of Mg+2 ion was lower than those of FW and CsW (P<0.05). The Hct, Hb, MCV, MCH, and plasma osmolarity were decreased with increasing salinity. The MCHC and plasma cortisol levels were increased with increasing salinity (P<0.05). The number of WBC and RBC did not change in different waters (P<0.05).
Based on our data and that of others17,15,10 there is a good similarity in Na+, K+, Ca+2, and Mg+2 ions concentrations between FW and CSW and adapted sturgeon, indicating an osmotic and ionic regulation.
After direct transfer of fish from FW to the CsW, the plasma Na+ concentrations of fish were determined to be higher than fresh water and less than the saline water. Also, Plasma Mg+2 concentrations (1.86±0.02 mEq/l) were determined much lower than Caspian Sea water (63.3±3.06 mEq/l) (Table 2). These results are similar to the results of other researchers on the plasma concentrations of adapted sturgeon in salt water.10, 17,22
Death is the ultimate biological consequence of environmental change. In fish that did not achieve ionic and osmotic compatibility under salinity stress in the Caspian Sea water, fish death occurred.23 In this survey, the survival rate with increased size (and age) was increased.
Some authors suggest that there is no correlation between body size and hematological parameters such as Hct, erythrocyte size and number, and hemoglobin concentration.24, 25 This is supported by the results of the present study in deferent size and age juvenile A. persicus. But, the Hct, MCV decreased and MCHC increased in FW compared to CSW (p<0.05), and Hb, MCH, RBC and WBC did not change (p>0.05), (Table 1).
Martinez -Álvarez et al.23 and Plaut indicate that these changes depend on the volume of water in the blood and are influenced by changes in environmental salinity.23
In this study, in the direct transfer of fish to brackish water, the number of fish blood cells did not change. But their red blood cell volume dropped. For this reason, this change can be related to the water volume of fish blood in brackish water. The results of this study are similar to with Tajan and Zohrabi research on some hematological and biochemical parameters of Cultured Stellate sturgeon (Acipenser stellatus) and Ship sturgeon (Acipenser nudiventris).26
The cortisol is the most important hormone in fish ion-osmoregulation in saltwater. Cortisol has been shown to influence the movement of sodium ions across the gill, in both fresh-water-adapted and seawater- adapted eels.27,28 The increase of plasma cortisol levels is considered to be a primary indicator of the stress response. In the present study, the trend of increasing levels of cortisol at higher salinity in juvenile A. persicus (Table 1) were similar to chondrostean.23,26,29
However, blood parameters and levels of cortisol, osmolarity, and ion concentration values in CsW did not return to initial values in FW. The result has been shown that the osmoregulation system caused physiological changes in this species. Therefore, it is possible to release juvenile sturgeon directly from FW to CSW with increasing weight and age.
As a result, due to the increasing pollution of rivers in the southern region of the Caspian Sea, it is not possible to release juvenile’s sturgeon into the river environment (fresh water). On the other hand, it is not possible to rehabilitate rivers in the current situation due to the need for high financial resources and the social conditions of the coastally people. For this reason, according to the results of this study, the juvenile’s sturgeon obtained from artificial reproduction in the sturgeon propagation centers of Iran can be released directly into the sea. In this method, the survival of the fish will increase with increasing weight and age.
The author declares that there are no conflicts of interest.
Iranian Fisheries Science Research Institute (IFSRI), Caspian Sea Ecology Research Center (CSERC)
We are grateful to all our colleagues in the Shaheed Marjani Sturgeon Propagation Center and Caspian Sea Ecology Research Center.

©2021 Farabi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.