Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 4
1Department of Marine Science Mawlamyine University Myanmar
2Director Marine Science Association Myanmar MSAM Myanmar
Correspondence: Soe Pa PaKyaw Department of Marine Science Mawlamyine University Myanmar
Received: June 24, 2018 | Published: August 23, 2018
Citation: Kyaw SPP, Soe-Htun U. Studies on the developmental morphology and life history in culture of Laurencia sp . 1 (Ceramiales, Rhodophyta) from Myanmar.J Aquac Mar Biol. 2018;7(4):246?251. DOI: 10.15406/jamb.2018.07.00215
The development of vegetative and reproductive structures and life history of Laurencia sp. 1 (Ceramiales, Rhodophyta) had been investigated. The detailed observations of the developmental morphology and life history were undertaken based on field-collected and cultured plants. The materials used in this study show the tetrasporangia originate from a pericentral cell near the growing apex. The sporangia show a parallel arrangement along the axis of the branch and adaxially from the pericentral cell. Mature tetraspores developed into a reddish brown colour upright shoot with colorless rhizoidal filaments. The gametophytic plants are dioecious. Spermatangia or fertile trichoblasts originate from an axial cell near the growing apex and consist of repeatedly dichotomously branched. The spermatangial branches cultured with cystocarpicbranches, and then ripe cystocarps formed. The mature cystocarp form a domoid-shaped pericarp with an ostiole, and release carpospores. These carpospores germinated and grew into erect shoot with colorless rhizoidal filaments. The morphology of Laurencia sp. 1 in Myanmar distinct from other related plants such as L. intricate Lamouroux and L. pygmaea Weber-van Bosse found in Asiatic water. The diagrammatic presentation of reproductive features in life history of Laurencia sp.1 was shown. This species shows the Polysiphonia-type tri-phasic life history.
Keywords: ceramiales, culture, development, Laurencia sp. 1, life history, morphology, Myanmar, rhodophyta
The genus Laurencia is a small to medium-sized red algae, under the order Ceramiales and family Rhodomelaceae. Laurencia species are mainly found in warm waters, and often tropical and subtropical parts of the world.1,2 These species grow in a variety of habitats such as, in tide pools, on reef flats, in protected or exposed locations, and on stones, rocks, dead coral, and other seaweeds of intertidal and subtidal zones. These species are characterized as 'turf-forming'. They can dominate the hard substrata in some marine communities.
Lamouroux established the genus Laurencia in 1813. In the first description of this genus, Lamouroux (1813) emphasized that Laurencia vary a lot; they differ equally in the diverse stages of their growth, and thus rendering their synonym very obscure. Harvey (1860) also noted that young plants may not resemble older ones. Yamada (1931) recognized that the external habit of the frond and the stichidial branches are very variable according to the conditions under which they grow (as cited in McDermid).3 According to Guiry and Guiry,4 the genus Laurencia has about 137 species. This genus is widely distributed in temperate and tropical seas.
The genus Laurencia is a small to medium-sized red algae, under the order Ceramiales and family Rhodomelaceae. Laurencia species are mainly found in warmer waters, and often tropical and subtropical parts of the world.1This species grow in a variety of habitats such as, in tide pools, on reef flats, in protected or exposed locations, and on stones, rocks, dead coral, and other seaweeds, of intertidal and subtidal zones. This species is characterized as 'turf-forming'. It can dominate the hard substrata in some marine communities.
Abe et al.,5 studied the development of tetrasporangia, spermatangia, procarp and cystocarpstructures in L. brongniartii from southern Japan. Masuda et al.,6 described the development of tetrasporangia, spermatangia, procarp and cystocarp structures in L.palisada from the Philippines. Masuda et al.,7 studied the formation of vegetative thallus, tetrasporangia, spermatangia and cystocarp in three species of Laurencia, L.majuscula(Harvey) Lucas, L.intricata Lamouroux, and L. perforata(Bory) Montagne from Canary Islands.
In Myanmar, SanTha Tun8 studied the laboratory culture of the red alga, Laurenciasp. collected from Setse coastal areas. He also described the optimal growth of thallus and sporelings in Laurencia sp. under various environmental parameters such as salinity, temperature, light intensity, photoperiod, and nutrients in the laboratory. Soe Pa Pa Kyaw9 reexamined and identified the plants of Laurencia as Laurencia sp. 1, Laurencia sp. 2, L. pinnata Yamada and L. composite Yamada, using the specimens collected from the three Coastal Regions of Myanmar from 1969-2014.
The objectives of the present study are (1) to know the development of vegetative and reproductive structures, and (2) to understand the complete life history of field-collected and cultured plants of Laurencia sp.1 belonging to the genus Laurencia.
The specimens were collected from the upper intertidal to subtidal zones from Nyawbyin(Lat. 14°08′ N, Long. 98°06′ E), Sitaw (Lat. 15°11′ N, Long. 97°48′ E), Kawdut (Lat. 15°32′ N, Long. 97°45′ E), Kalagoke (Lat. 14° 45′N, Long. 97°30′ E), Setse (Lat. 15°52′ N, Long. 97°35′ E), Kyaikkhami (Lat. 16°05′ N, Long. 97°34′ E), Mawtin point (Lat. 16°04′ N, Long. 94°20′ E), Ngapali (Lat. 18°21′ N, Long. 94°21′ E) and Sittwe (Lat. 20° 08′ N, Long. 92°54′ E) along the three Coastal Regions of Myanmar from June 2010 to January 2014 (Figure 1). The collected plants were brought to the Phycological Research Laboratory in the Department of Marine Science, Mawlamyine University, Mawlamyine, in ice-boxes. In the laboratory, the fresh and healthy plants were thoroughly washed with painting brushes in the sterile seawater to remove epiphytes and some contaminants. Then, all these plants were kept in glass jar containing the sterile seawater under white fluorescent tube (Light intensity of 100-200 ft-c=20-40µmol m-2s-1) at room temperature for further study. For the culture experiments, seawater was filtered with Whatman No. 1 filter paper and then autoclaved. Natural seawater was collected from Setse coast, and then stored in plastic drums placed in cold and shaded place. After that, it was heated for the higher concentration of salinity and diluted with distilled water for the lower concentration of salinity.
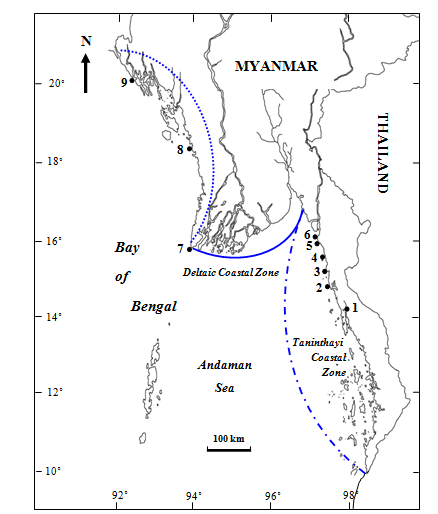
Figure 1 Map showing the collection sites of the Laurencia sp. 1. 1. NyawByin, 2. Sitaw, 3.Kawdut, 4.Kalagoke, 5.Setse, 6.Kyaikkhami, 7.Mawtin Point, 8.Ngapali, 9.Sittwe.Taninthayi; Deltaic; Rakhine.
For the experiments, fresh and healthy plants were selected and used as seed materials. The apical tips of sterile (vegetative) and fertile (spermatangia, cystocarpic and tetrasporangia) branches were cut into pieces of 1.5mm in length by hand using sterilized double-edged razor blades. The apical tips measured 1.5mm in length were inoculated into Petri dishes containing 20ml of seawater in 20‰ with Provasoli's Enriched Seawater (PES) medium.10 1mg per liter germanium dioxide (GeO2) solution was used for eliminating diatoms, and antibiotic stock solution (penicillin and streptomycin) was also used for killing bacteria and blue-green algae. The culture medium was replenished once a week. During the experimental period, all the culture apparatuses were autoclaved.
The cultures were kept at 25°C under 16:8h light/dark cycle and light intensity of (70-240ftc=15-50µmolm-2s-1) in Gallenkamp Incubators (Volts 220/240, Hz 50, Cat No. INF 781-T). Measurements of light were taken by a light meter (Triple Range 214) and salinity was measured by using a salinity refractometer (Atago Cat. No. 2493 Japan). In this study, culture experiments were repeated three times.
The fragments of cultured plant were squashed on microscope slides, and stained with aniline blue (0.5g water soluble aniline blue in 100ml distilled water and 5ml conc. Acetic acid) and acid fuchsin(1% solution in distilled water and 70% alcohol) for detailed observations. The sections were prepared manually by hand using razor blades. They were analyzed under a compound light microscope (Olympus 285872, Nikon, Japan), and an electrified microscope (Olympus U-APT, T5 SN 9D 06211, Japan). Microscopic measurements were recorded in micrometer (µm) using the ocular meter. External and internal morphological structures were photographed with a Panasonic (Lumix) DMC-TZ 15 digital camera and processed using Adobe Photoshop CS. As for classification study basically followed the classification system used by Saito,11,12 Saito & Womersley13 and Guiry & Guiry.4
(a) A classification system of the genus Laurencia
The classification system basically follows Saito,11,12 Saito & Womersley13 and Guiry &Guiry.4
Phylum: Rhodophyta
Class: Florideophyceae
Order: Ceramiales
Family: Rhodomelaceae
Genus: Laurencia Lamouroux 1813: 130
Section: Laurencia
Species: Laurencia sp. 1
(b) Habitat and morphological notes of field-collected plants, Laurencia sp.1 (Figures 2-6)

Figures 2-6 Field-collected plant of Laurencia sp.1. (2) Habit of a plant. (3) Part of a sterile plant. (4) Part of a tetrasporangial plant. (5) Part of a spermatangial plant. (6) Part of a carpogonial plant.
Plants grow on mud flat or on rocks covered with sand in the upper to lower intertidal zones. This species attached to the substratum by stoloniferous holdfast. It occurs in places exposed to wave action forming dense carpets, and grows together with Schizothrixsp., Cladophora sp., C. rupestris, C. vagabunda, Chaetomorphagracilis, C. linum, Dictyotaadnata, Gelidiumcrinale, G. arenarium, Catenellanipae, Ceramium sp., Caloglossabengalensis, Polysiphoniasubtilissima, Acanthophoraspicifera, Bostrychiatenella, B. binderi. The plants can be found throughout the year. The luxuriant growth found in early August to late October. Spermatangial plants appear in early August and late September. Mature cystocarpic plants can be seen from mid-Augustto late October. Mature tetrasporangial plants are found from late July to late October.
Fronds, caespitose and prostrate (Figure 2), 3-11cm in diameter, densely entangled with stoloniferous branches, reddish brown, rigid, and cartilaginous in texture, attached to the substratum by discoid holdfasts formed on main axes and secondary branches; main axes, terete with blunt tips terminate in a small depression; branches cylindrical (Figure 3), 2-12mm long, irregularly alternate. Trichoblasts grow from a young pericentral cell near the growing apex of the apical depression; repeatedly dichotomously branched.
The cortical cells are subquadrate, not protrude at the apex and not form a palisade-like layer, with secondary pit connections between cortical cells. The Corpsen cerise are absent in cortical and sterile trichoblast cells. Lenticular thickenings in the walls of the medullary cells are absent and axial cell with four pericentral cells.
Tetrasporangial stichidia (Figure 4), formed on the apical portions of secondary branches and branchlets; simple or with 1-5 branches; cylindrical, 725-2000µm long and 500-700µm broad. Tetrasporangia, tetrahedrally divided; adaxially from the pericentral cell, and with parallel arrangement. Plants, dioecious; spermatangial stichidia(Figure 5), formed on the apical portions of main axis; slightly turbinate, single or in clusters, 250-625 µm in diameter when young; cylindrical with distinct stipe, simple or with 1-3 branches, 1.2-2.6mm long, and 0.7-1.1mm broad when mature; with apical cup-shaped spermatangial pits, and contain numerous fertile trichoblasts. Trichoblasts, arise from a young pericentral cell near the apical cell; consist of a repeatedly dichotomously branched; terminate in a single large sterile vesicular cell. In the carpogonial plant (Figure 6), the secondary branches and branchlets cylindrical when sterile, but these become broad with the development of the cystocarps. Cystocarps, single or in clusters(1-5 lobes) with one or more apical ostiole, borne laterally on branches (except first to fourth-order branches), broadly ovoid, slightly pointed at these apices; 570-1100µm long and 670-1800µm broad at maturity. The terminal cells of the gonimoblast increase in size and become carpospores.
(c) Developmental stages and life history of Laurencia sp.1
(i) Development of vegetative structure (Figures 7-19)

Figures 7-13 Laurencia sp.1 cultured at 25°C under 16:8 LD. (7-9) Apical portion of main axis with lateral branchlets(MMB 11136). (10) Cross section of main branch with medulla (m) and cortex (c) cells (MMB 11137). (11) Longitudinal section of main branch with medulla (m) and cortex (c) cells (MMB 11138). (12-13) Cross section of main axis with initial lateral branch (MMB 11139).
Many short adventitious branchlets(Figures 7-8) are produced on the apical portion of main axis and the first order branches. These branchlets are simple. Branches (Figure 9) are irregularly arranged in manner. The branching pattern of cultured plants is differed from field-collected plants. Transection of main axes and branches (Figures 12-13) of Laurencia sp. 1 shows fully-developed parenchyma cells. The apices exhibit a group of 4 to 7 apical cells. The lateral axes arise from these apical cells. These cells are compacted into the cortex, which shows large inner cells and small external ones.
Laurencia sp. 1 is corticated. Secondary pit connections are formed between small cortical cells and linked with the pericentral cell below. Growth is from an apical cell which is located in a terminal pit (Figure 15). The single axial filament is usually clearly evident at maturity, centered in a parenchymatous tissue of large, medullary cells and small cortical cells (Figures 10-11). The main axes of the cultured plants have four pericentral cells (Figure 14) per axial cell.
The trichoblast (Figures 16-17) grow from a young pericentral cell, which is located near the apical cell. The first branches produce laterals alternately. The monosiphonoustrichoblasts taper from the base to apex and consist of (4-10) branches, each terminating obtusely with a conical cell. The mature trichoblasts are subdichotomously branched. The formation of corps en cerise was not found in cortical and sterile trichoblast cells.
The unicellular rhizoids (Figures 18-19) (200-750)µm grow from the pericentral (inner or medullary) cells of the lower portions of a branch. When these rhizoids come in contact with the substrate, they produce a disc-shaped holdfast. Creeping or stolon-like branches are formed.
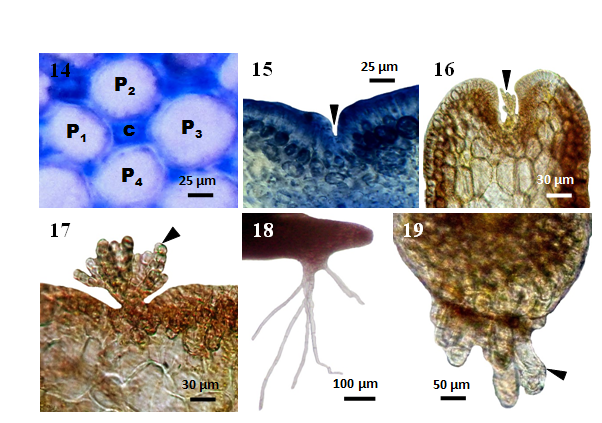
Figures 14-19 Laurencia sp.1 cultured at 25°C under 16:8 LD. (14) Cross section of main branch showing central (c) and four pericentral (p) cells (MMB 11140). (15) Longitudinal section of main branch showing apical depression (arrowhead) (MMB 11141). (16) Longitudinal section of main branch showing apical depression with initial trichoblast (arrowhead) (MMB 11142). (17) A young trichoblast (arrowhead) (MMB 11143). (18) Unicellular rhizoids (MMB 11144). (19) Longitudinal section of main branch showing unicellular rhizoids (arrowhead) arising from the pericentral cells (MMB 11145).
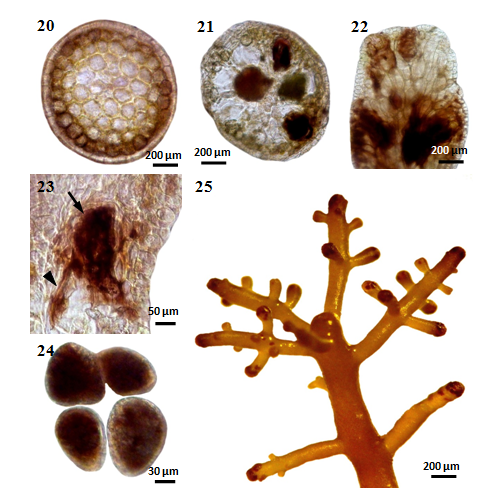
Figures 20-25 Laurencia sp.1 cultured at 25°C under 16:8 LD. (20) Cross section of initial tetrasporangialstichidium (MMB 11146). (21) Cross section of mature tetrasporangialstichidium (MMB 11147). (22) Longitudinal section of tetrasporangialstichidium showing parallel arrangement of tetrasporangia (MMB 11148). (23) Longitudinal section of tetrasporangialstichidium showing a tetrasporangium (arrow) on an elongated pericentral cell (arrowhead) (MMB 11149). (24)Tetrahedrally divided tetrasporangia (MMB 11150). (25)Tetrasporangialbranchlets formed on the plant (MMB 11151).
The vegetative cells (Figure 20) are directly transformed into tetraspores. Thetetrasporangium(Figure 21) originates from a pericentral cell near the growing apex in the apical depression of a branchlet. The tetrasporangia are tetrahedrally form divided (Figure 24). It is in the medullary tissue of mature plants. The tetrasporangium initial is cut off from an elongated periaxial cell (Figure 23) towards the adaxial side. The sporangia show a parallel arrangement (Figure 22) along the axis of the branch. Thetetrasporangia are confined to stichidium-like fertile ultimate branchlets (Figure 25) and measured 250-1675µm long and 200-300µm broad in size in surface view.
The spermatangial stichidium (Figures 26-28) are thick and 350-1150µm long and 580-650µm broad. The stichidium has broad apical depressions which are furnished with numerous sterile and fertile trichoblasts and are called spermatangial depressions. These depressions consist of cup-shaped cavities (Figures 29-30) in which the spermatia originate from fertile cells. The fertile trichoblast (Figures 31-32) originates from an axial cell near the growing apex and consists of a repeatedly dichotomously branched and terminate in a single large sterile vesicular cell.
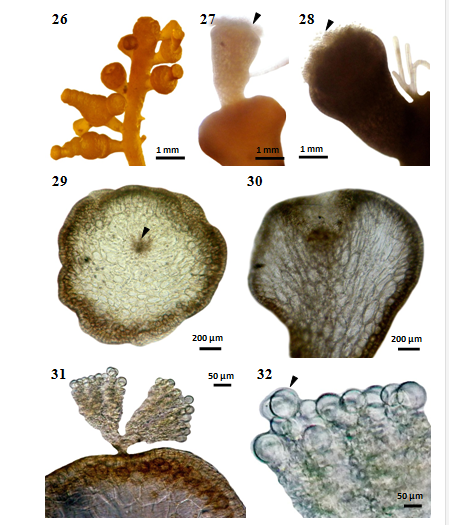
Figures 26-32 Laurencia sp.1 cultured at 25°C under 16:8 LD. (26)Spermatangial branchlets formed on the plant (MMB 11152). (27-28) Spermatangialstichidia with fertile trichoblasts (arrowhead) (MMB 11153). (29) Cross section of spermatangialstichidium with fertile trichoblast(arrowhead) (MMB 11154). (30) Longitudinal section of spermatangialstichidium showing a cup-shaped spermatangial depression (MMB 11155). (31) Cross section of spermatangialstichidia with fertile trichoblasts (MMB 11156). (32) Mature spermatangial with sterile vesicular cell (arrowhead) (MMB 11157).
The initial cell of the female reproductive organ (cystocarp) forms from a pericentral cell of the fertile branchletand acts as the fertile central cell of the procarp. Procarps(Figure 33) are formed in apical pits of fertile branches but they situated below pits by apical growth. The apical portion of carpogonial branch (Figure 34) is provided with a long slender trichogyne. The trichogyne does not pass directly to the surface of the thallus. The carpogonial filaments are four-celled and are initiated near the apex of fertile branches. The four cells of the carpogonial filament stain deeply and lie in a plane. The procarps consist of auxiliary cell bearing a supporting cell. The auxiliary cells are large and irregular in shape, and have granular, deeply staining contents. The supporting cell then fuses with the fertilized auxiliary cell to form a fusion cell. The mature cystocarp contains a large central fusion cell (Figures 35-36) and radiating gonimoblast filaments (Figure 37), each of which terminates in an elongate carposporangium. There was only a single carposporangium at the tip of each gonimoblast filament. The gonimoblast filaments develop towards the thallus surface, and the mature cystocarp (Figure 38) form a domoid-shaped pericarp with an ostiole. The cortical cells of the surrounding thallus serve as the wall of the cystocarp. When the cystocarp matures, the cortical filaments in part of the wall separate to form an ostiole. The fertile branchlets (Figure 39) of carpogonial plant are cylindrical while young, but becoming clavate with the development of the procarp and cystocarp.
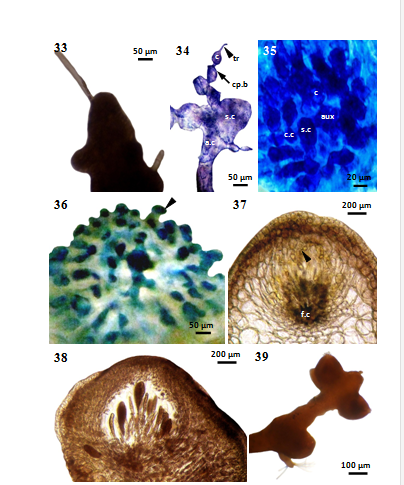
Figures 33-39 Laurencia sp.1 cultured at 25°C under 16:8 LD. (33)Procarp (MMB 11158). (34) Longitudinal section of procarp, showing four-celled carpogonial filament and supporting cell (MMB 11159). (35) Cross section of cystocarp, showing the initial stage of fusion cell formation (MMB 11160). (36) Longitudinal section of cystocarp, showing an early stage of gonimoblast (arrowhead) formation (MMB 11161). (37) Cross section of cystocarp, showing the fusion-cell and initial stage of gonimoblast filament (arrowhead) (MMB 11162). (38) Longitudinal section of a ripe cystocarp (MMB 11163). (39)Cystocarps formed on the plant (MMB 11164). a.c= axial cell, aux=auxillary cell, c.c= central cell, c= carpogonium, cp.b=carpogonial branch, f.c=fusion cell, s.c= supporting cell, tr= trichogyne.
Figure 40 represents the diagram of reproductive features in life history of Laurencia sp.1, according to the stages of the development of reproductive structures, viz., tetrasporangia, procarp, cystocarp and spermatangia and the spore germination. The germination pattern of tetraspore was similar in carpospore. The germlings of tetraspore and carpospore showed the Fucus type germination pattern. According to the stages of the development, the spermatangialstichidia released male gametes or spermatia. And then, male gametes or spermatia fused with the female gametes or carpogonia. Carpospores were released after mixing with male gametes or spermatia and female gametes or carpogonia. Carpospores germinated into tetrasporophytic plants. Tetraspores released and then germinated that formed male and female gametophytic plants.
The closely related species of Laurencia sp. 1 was probably L. intricate Lamouroux. Its type locality was the Antilles and Cuba.4 Authentic material of L. intricate Lamouroux had not been available for comparison. The vegetative and reproductive characters of Laurencia sp. 1 were compared through the data from the available literatures. This species is newly recorded from Myanmar waters.
The present study was undertaken based on field-collected and cultured plants to evaluate the vegetative and reproductive features and to understand the life history. The field-collected and cultured plants of L. sp. 1 were essentially the same as anatomically, but different in the formation of branching pattern. The main axes of the field-collected and cultured plants have four pericentral cells per axial cell. The branching patterns of the cultured plants were irregularly arranged in manner, whereas field-collected plants in repeatedly dichotomously branched.
Moreover, the stages of the development of reproductive structures, viz., tetrasporangia, procarp, cystocarp and spermatangia, were observed in cultured experiments. The formation of tetrasporangial and spermatangialstichidia of the cultured plants was somewhat similar in those of field-collected plants, but different in cystocarps formation. Cystocarps of cultured plants are formed on the apical portions of main axis and laterally on first to second-order branches, whereas field-collected plants borne laterally on branches (except first to fourth-order branches).
The sporangia of L. sp. 1 have anarrangementparallel along the axis of the branch in field-collected and cultured plants. The parallel arrangement of the tetrasporangia was regarded by Saito11 as a characteristic feature of the genus Laurencia. In the procarp of L. sp. 1, the carpogonial branch has four-celled and borne on a large supporting cell. The result of this study coincides with all Laurencia spp. in Japanese specimens.11 The spermatangial depressions consist of cup-shaped cavities which are furnished with numerous sterile and fertile trichoblasts. The fertile trichoblast consists of repeatedly dichotomously branched and terminate in a single large sterile vesicular cell. The trichoblast type of spermatangia was regarded by Nam, Maggs and Garbary14 as a characteristic feature of the genus Laurencia.
The development of vegetative and reproductive structures and life history of Laurencia sp. 1 (Ceramiales, Rhodophyta) had been investigated using the material collected from NyawByin, Sitaw, Kawdut, Kalagoke, Setse, Kyaikkhami, Mawtinpoint, Ngapali and Sittwe Coastal areas of Myanmar. The detailed observations of the developmental morphology and life history were undertaken based on field-collected and cultured plants. In cultured and field-collected plants of Laurencia sp. 1, male and female gametophytic plants are present separately. Male gametophytic plant bears spermatangia on male trichoblast, whereas the female gametophytic plant bears carpogonia. Spermatangia bear male gametes or spermatia whereas carpogonia bears female gamete. The male and female gametes fuse. Inside the carposporangia develops a carpospores. This carpospores germinates and forms the tetrasporophytic plant. Tetrasporophytic plants develop tetrasporangia. In a life-cycle, the haploid phase is represented by male and female gametophytic plants. The first diploid phase is represented by carposporangia and carpospore. The second diploid phase is represented by tetrasporangia. Hence, this life-cycle has three stages (triphasis). Because of one haploid phase (male and female gametophytic plants) and two diploid phases (carpospore and tetraspore) are present. So, Laurencia sp.1 shows the Polysiphonia-type tri-phasic life history.
None.
Author declares that there is no conflict of interest.

©2018 Kyaw, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.