Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 3
Laboratory of Ichthyology Zoological Institute Russian Academy of Sciences Russia
Correspondence: Mikhail Yur evich Zhukov Laboratory of Ichthyology Zoological Institute Russian Academy of Sciences Russia, Tel 007-(812)-328-06-12
Received: June 13, 2018 | Published: June 26, 2018
Citation: Zhukov MY. Some characteristic features of the axial skeleton of Zanclorhynchus ( Zanclorhynchinae: Congiopodidae ) off Crozet Islands, Southern Ocean. J Aquac Mar Biol. 2018;1(3):172 ?175 DOI: 10.15406/jamb.2018.07.00204
The goal of this work is to describe previously unpublished data about axial skeleton of Zanclorhynchus, one of the most numerous, but still poorly known genus from the islands of the Indian sector of the Southern Ocean.
Keywords: Zanclorhynchus, axial skeleton, Southern Ocean
According to the latest data1, the genus Zanclorhynchus Günther, 1880, includes two species: Zanclorhynchus chereshnevi Balushkin et Zhukov, 2016 and Z.spinifer Günther, 1880 with two subspecies–nominative Z. spiniferspinifer Günther, 1880 and Z. spiniferheracleus Zhukov Balushkin, 2018.
Despite the fact that in a number of cases Zanclorhynchus dominate in number and constitute a significant part of the dietof top predators, the genus still remains weakly studied. Until recently, only a few articles were devoted to the anatomy of that fishes,2–4 in general information is limited to inclusion in lists with a brief description.5
Zanclorhynchus have highest values in number (approximately2/3 of the total catch of trawling) on the shelf of the Kerguelen Archipelago with a density of more than 10000sps/km2, the largest numbers are recorded in the northwest of the islands at a depth of 200-1000m6. On the Macquarie shelf Zanclorhynchus is the second (24.8%) in number species.7 On the Kerguelen Archipelago Zanclorhynchus is in the diet of the Gentoo penguin Pigoscelispapua,8 also these penguins feed on Zanclorhynchus near the island of Macquarie - constitute almost 40% of the diet.9 They enter the diet of the Porbeagle Lamnanasus on the Kerguelen Archipelago (CherelandDuhamel 2003). On the Heard Island otoliths of Zanclorhynchus were found in the faecal material of the Antarctic fur seals Arctocephalus gazella11. Is a favorite food among the ichthyofauna of the Hooker’s sea lions Phocarctoshookeri off Macquarie Island (Mc Cahon et al. 1999).12In the same area the Rockhopper Penguin Eudypteschrysocome also feeds on Zanclorhynchus.13
The axial skeleton was studied under X-ray images obtained on a PRDU-02 X-ray diffractometer. Were studied 44 specimens of Zanclorhynchus: ZIN no. 40248–26 specimens, SSS“Skif”, cruise no. 3, stationno.944/33, trawl no. 18, Crozet Islands, 45°51ʹ S, 49°47ʹE, depth 235-260м, 04.12.1970, collector A.F. Pushkin; ZIN no. 45675– 4specimens, MFFT “Aelita”, stationno. 41, Crozet Islands, 45°52ʹ S, 49°54ʹE, depth230 м, 25.01.1968, collectors A.I. Karpenko and G.S. Volya; ZIN no. 5630–14specimens, sampled in the same trawl with ZIN no. 40248.
The number of vertebrae is 33 - 36, including urostyle. In the overwhelming majority of cases (94%) the number of vertebrae is 34(48%) or 35(46%).
The number of proximal radials before D2 strictly corresponds to the number of vertebrae +1. The proximal radial, serving as a support, and not only associated with the fin ray through the distal and middle radials, is considered to be the corresponding to the ray.13 A double number of proximal radials between the first and second neural spines is explained by the loss of the first vertebrae (one or two), which is considered an autapomorphy among the super family Scorpaenoideasensu Imamura 2004.3,14 About 2/3 of the studied Zanclorhynchus have proximal radials (free between D1 and D2, either corresponding to ray at the end of D1 or at the beginning of D2), entering in double between neural spines. In this case, not far (up to 6 vertebrae, usually 1 or 2), there is necessarily a pair of neural spines without aninterneuralia between them (Figure 1).
One specimen was found to have no free proximal radials between the dorsal fins (Figure 2), and the last spiny ray D1 is supported by two interneuralia. This mistake in ontogenesis, highly likely, led to the disappearance of the subsequent interneuralia. The normal development of proximal radials in the second dorsal fin is due to the presence of several segmentation centers in ontogenesis. In Teleostei, there are three main centers: the "occipital", from which the segmentation goes back; "central" - the differentiation of the vertebrae goes in both directions and "caudal" with the development of the vertebrae in the rostral direction13. Here we can see the presence of the "occipital" center, the error in which led to the absence of free proximal radials between dorsal fins, and the "central" segmentation center that caused the normal development of D2 (the first ray of which have no corresponding radial yet).
Before the anal fin there usually is free interhaemalia, which enters between the haemal spines of the first two caudal vertebrae is connected with the first ray of the anal fin through the distal and middle radials (Figure 3A), the corresponding ray is absent or extremely reduced (Figure 3B), the normal ray is developed on the first interhaemalia in 4% of cases. Usually the first ray of the second dorsal fin is shortened, sometimes reduced so that it can be seen only on the X-ray image (Figure 3C), probably it could completely disappear in similar way as the first ray of the anal fin.
The last proximal radial in the anal and second dorsal fins is shortened and expanded, the so-called "stay". In about 80% of cases, it serves as a support for the last two shortest rays, in a 20% "stay" supports only one. Of the last three interneuralia and interhaemalia, often (about 60% of cases), two of them double enters between the spines of both the neural and haemal (Figure 4).
In the caudal fin, there are strictly 12 principal nonbranched segmented rays, 6 rays on each hypural plate, upper and lower. Two epuralia take part in support of two marginal upper rays, others upper marginal rays (0–4) supports by last neural spine. 1–2 lower marginal rays supports by last haemal spine. All margin rays are nonbranched and non segmented. The first preural vertebra (pu) in about 10% of the Zanclorhynchus has doubled spines, neural and/or haemal (Figure 5A) (Figure 5C). In nototheniids, for example, the complex structure of the first preural vertebra appeared by the separation of the originally unified structure13. Detection of the specimen ZIN no. 56301 (3) with separate vertebrae (Figure 5B) (Figure 5D) allows us to surely conclude that a complex first preural vertebra appeared by merging of two vertebrae. The existence of such an individual reversion can indicate the phylogenetic youth of this merging.
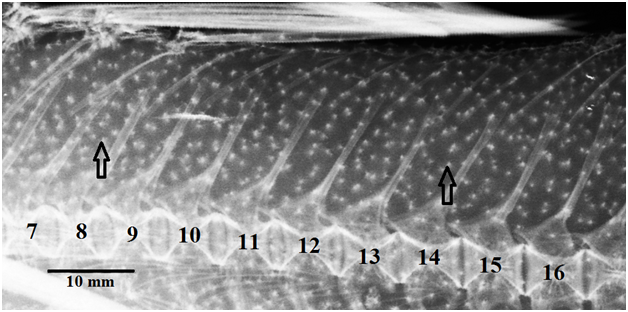
Figure 1 X-ray image of the area between two dorsal fins ZIN no. 40248 (21). Numbers indicate the number of the vertebrae (16th vertebra - 1stcaudal), arrows - an empty gap between the neural spines and a gap with pair of incoming inter neuralia.

Figure 2 X-ray image of the area between two dorsal fins ZIN no. 40248 (22). Numbers indicate the number of the vertebrae (16th vertebra - 1stcaudal).

Figure 3 X-ray images of the rostral parts of anal and second dorsal fins, numbers indicate the number of the vertebrae (A–17th vertebra = 1stcaudal, B and C–16th vertebra=1st caudal), arrows indicates extremely reduced rays; A–anal fin ZIN no. 56301 (7) with first free interhaemalia, hs–haemal spine, pr–proximal radial, mr– middle radial, dr–distal radial; B–second dorsal fin ZIN no. 56301 (14); C–anal fin ZIN no. 45675 (2).
The study was supported by the State Research Program no. AAAA-A17-117030310197-7.
The author is thankful to the head of the Laboratory of Ichthyology ZIN RAS Balushkin A. V.
The author declares that there is no conflict of interest.

©2018 Zhukov. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.