Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 3
1Research Scientist, Marine Science Association, Myanmar (MSAM), Myanmar
2Professor and Head, Department of Marine Science, Pathein University, Myanmar
3Flinders University of SA, Australia
4Manager, Marine and Freshwater Program, Fauna and Flora International-Myanmar Program, Myanmar
Correspondence: Thu-Rein, Research Scientist, Marine Science Association, Myanmar (MSAM), Myanmar
Received: May 16, 2019 | Published: June 26, 2019
Citation: Thu-Rein, Aung C, Murray-Jones S, et al. Seagrass surveys in Shwe Thaung Yan coastal areas, the southern part of Rakhine Coastal Region, Myanmar: biodiversity, coverage and biomass. J Aquac Mar Biol. 2019;8(3):105-112. DOI: 10.15406/jamb.2019.08.00248
Studies on percent cover and biomass of seagrasses from Shwe Thaung Yan coastal areas (Inn Din Gyi, Kyauk Nagar and Phoe Htaung Gyaing), the Southern parts of Rakhine Coastal Region, were carried out between March and August, 2018. A total of 8 species of seagrasses, namely Syringodiumisoetifolium (Ascherson) Danty, Halodulepinifolia (Miki) den Hartog, Haloduleuninervis (Forsskal) Ascherson, Cymodocearotundata Ehrenberg et Hemprich ex Ascherson, C. serrulata (R. Brown) Ascherson et Magnus, Thalassiahemprichii(Ehrenberg) Ascherson, Halophila major (Zoll.) Miquel and Enhalusacoroides (Linnaeus f.) Royle, were recorded in three study sites. Seagrass meadow in this study showed seasonal variations in both percent cover and biomass. Total seagrass coverage and biomass were higher in the dry season than in the monsoon season. Total seagrass coverage ranged between 8% and 75% in Phoe Htaung Gyaing, between 10% and 42% in Kyauk Nagar, and between 15% and 43% in Inn Din Gyi. Total seagrass mean biomass was 50.2413-259.846gdry.wtm-2 in Phoe Htaung Gyaing, 63.0194 -321.535gdry.wtm-2 in Kyauk Nagar, and 98.6819-416.237gdry.wtm-2 in Inn Din Gyi.
Keywords: Biomass, Coverage, Cymodocearotundata, serrulata, Enhalusacoroides, Halodulepinifolia, Haloduleuninervis, Halophila major, Syringodiumisoetifolium, Shwe Thaung Yan coastal area, Southern Rakhine Coastal Areas, sea grasses, Thalassiahemprichii
There are about 72 species of seagrasses worldwide,1,2 among which 16 species in Southeast Asia.3 Recently, Soe-Htun et al.4 reported a total of 11 species of seagrasses along the three Coastal Regions of Myanmar, namely Rakhine Coastal Region, Ayey arwady Delta- Gulf of Mataban Coastal Region and Tanintharyi Coastal Region.5 Seagrasses often occur in vast meadows and provide nurseries, shelters and foods for a variety of commercially, recreationally, and ecologically important species (e.g., fishes, sea turtles, dugongs, manatees, seahorses, crustaceans).6
Additionally, seagrasses filter nutrients and contaminants of estuarine and coastal waters, closely linked to other communities: in the tropics to coral reef systems and mangrove forests, and in temperate waters to salt marshes, kelp forests, and oyster reefs.7,8 Seagrasses trap sediments producing a reduction in wave motion and causing suspended particulate to fall out. Trapping sediments benefit corals by reducing sediment loads in the water. Mangroves trap sediments from land, reducing the chance of seagrasses and corals being smothered.9
Seagrass meadows globally are closely linked with high fisheries production, principally due to their values as a critical nursery habitat in all regions of the world,10–12 as well as their direct value for fisheries exploitation.13 There are a variety of factors that influence seagrass meadows in biomasses, distribution and species composition, such as physical disturbance, grazing, intraspecific competition, nutrients pollution and sediment laden flood waters.14–17 Combination of these parameters will permit, encourage or prevent seagrass meadows thriving.18 Pollution by industrial or intensive agricultural practices is the main threat to seagrass beds. Illegal and unsustainable fishing practices also threaten them with physical damages, including bivalve fishing that can be especially harmful.1 Dugongs have been reported along the Rakhine and Tanintharyi Coastal Regions due to the presence of a lot of the seagrass beds for feeding,19,20 but not in the Ayeyawady Delta which does not support the seagrass meadows due to hypo-saline conditions.21
The objectives of this research are:1) to investigate the species composition and 2) to access seasonal changes of percentage cover and biomass of seagrass beds under different physical parameters in Shwe Thaung Yan coastal areas, the Southern part of Rakhine Coastal Region, Myanmar.
Study sites
This research was conducted in three study sites of Shwe Thaung Yan coastal area (Figure 1), located in southern part of the Rakhine Coastal Region, namely Inn Din Gyi (IDG;17.06978˚N, 094.44969˚E), Kyauk Nagar (KNG; 17.09247˚N, 094.45264˚E), and Phoe Htaung Gyaing (PHG;17.16454˚N, 094.49873˚E), from March to August, 2018.These areas are ecologically very important in having valuable resources such as mangrove forests, seagrass meadows and patchy corals, providing feeding and nursery grounds for endangered species of dugongs and sea turtles.
Taxonomic studies
This research was carried out only during low tides when the seagrasses were exposed to air in PHG but the entire seagrass meadows of IDG and KNG were always submerged (even lowest tide). For taxonomic and distribution studies, fresh and live materials of seagrasses growing in the three study sites were collected by uprooting the seagrasses with a small knife from March to August 2018. The samples were initially washed, cleaned and preserved in 5% formalin in seawater. Samples of seagrasses were examined mainly on the vegetative characters, and then pressed on herbarium sheets to prepare as voucher specimens for each locality. All specimens were identified using the standard monograph of seagrasses prepared by Den Hartog22 and Kuo and Den Hartog.23 This study followed the classification system used by Fortes.24 All voucher specimens were deposited in the Herbarium of the Department of Marine Science, Pathein University (MSPT), Myanmar.
Monitoring on seagrass meadows
Snorkeling equipments were used for the submerged habitats of seagrasses growing in IDGsub-tidal zone and in depths between 1-2m, in KNG. This research followed the methods of SeagrassNet Manual by Short et al.,8 consisting of three fixed, parallel, 50m cross-transects referred to as cross-transects A, B and C, with cross-transect A, closest to shore, B, midpoint and C, most seaward of these cross-transects were established on a transect laid out seaward, perpendicular to the shore. Percentage cover of seagrasses (Coverage of seagrasses) was visually estimated within 12 randomly placed 0.25m2 quadrats along each transect line, using a photo guide of percent cover. Seagrass species composition and coverage were determined along all the cross transect and by measurement of cover in the quadrats, respectively. The physical parameters such as temperature by using a digital thermometer and chemical parameter, namely salinity measured by using a refractometer in the field were recorded.
For biomass studies, 108 core samples were monthly collected from 3 sites (for each site, 36 core samples were collected from the three transects), using 0.006204m² core (PVC core sampler) for small and medium-sized seagrasses. A biomass core is taken to 10cm depth outside each quadrat by selecting an area approximately 0.5m landward of the quadrat. Place the core over the selected shoots, making sure that leaves rooted in the core are on the inside of the core and those rooted outside are outside of the core. Wash the sediments from the core sample, separate into leaves, stems, and root-plus-rhizome. When processing, scrape epiphytes from the leaves and rinse the leaves in 5% formalin if epiphytes are present. Parts of seagrasses were rinsed in freshwater and each separated samples were weighed (wet weight) and dehydrated using oven (dry to constant weight) and immediately weighed again for dry weight by using digital balance. All biomass data were expressed gdry.wtm-2.8
A total of eight species of seagrasses collected from three study sites of Shwe Thaung Yan, in the southern Rakhine Coastal Region during study period were identified as Syringodiumisoetifolium (Ascherson) Danty, Halodule. pinifolia (Miki) den Hartog, Haloduleuninervis (Forsskal) Ascherson, Cymodocearotundata Ehrenberg et Hemprich ex Ascherson, C. serrulata (R. Brown) Ascherson et Magnus, Thalassiahemprichii(Ehrenberg) Ascherson, Halophila major (Zoll.) Miqueland Enhalusacoroides (Linnaeus f.) Royle (Figures 2-9).
Table 1 showed monthly species composition and abundance of seagrasses in three study sites from March to August, 2018. In IDG, Cymodocearotundata was the dominant species in transect A, whereas Cymodoceaserrulata was the dominant species in transect B and Thalassiahemprichii was in transect C. In KNG, Cymodoceaserrulata was the most dominant species in transect A, transect B and transect C. In PHG, Haloduleuninervis, was the dominant species in transect A, Halodulepinifolia was the dominant species in transect B and transect C (Figure 10).
|
ID |
KNG |
PHG |
||||||||||||||||
Sr.No |
Species |
March |
April |
May |
June |
July |
August |
March |
April |
May |
June |
July |
August |
March |
April |
May |
June |
July |
August |
1. |
Syringodiumisoetifolium |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
- |
- |
- |
2. |
Halophila major |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
3. |
Cymodocearotundata |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
4. |
C. serrulata |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
5. |
Haloduleuninervis |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
6. |
H. |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
7. |
Enhalusacorodies |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
8. |
Thalassiahemprichii |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Total number of species |
6 |
6 |
6 |
6 |
6 |
4 |
6 |
6 |
6 |
6 |
6 |
6 |
7 |
7 |
7 |
7 |
7 |
7 |
|
|
Symbol: +, present; -=absent |
|||||||||||||||||||
Table 1 Monthly species composition and abundance of seagrasses in three study sites of Myanmar coast, from March to August, 2018.
Symbol: +, present; -=absent
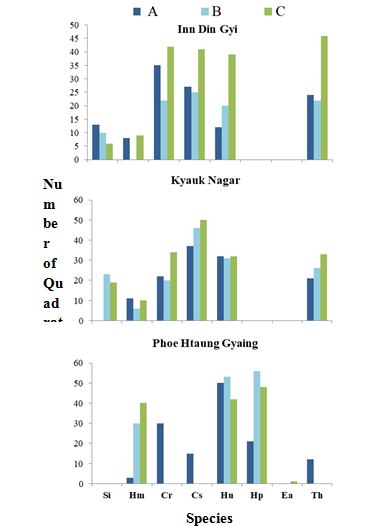
Figure 10 Dominant species along each transect in three study sites of Myanmar coast, from March to August, 2018.
Therefore, Cymodocearotundata, C. serrulata and Thalassiahemprichii were the most dominant species in IDG. Cymodoceaserrulata was the most dominant species in KNG, and Haloduleuninervis and Halodulepinifolia were the most dominant species in PHG. Enhalusacoroides and Halodulepinifolia were only found in PHG. Syringodiumisoetifolium was found in IDG and KNG, but not in PHG. Figure 11 showed the environmental variables of seagrass meadows in the sites studied.
In IDG, seagrass coverage (%) in transect C was the higher than transect A and B during the study period. In KNG, the coverage in B was higher than in A and C, in May, and in June and July, coverage in transect C was higher than in A and B; while it was lower in A than in B and C, during the study period. In PHG, the coverage in A was higher than in B and C, in March, April, July and August, and it was higher in B than in A and C in May and June (Figure 12).

Figure 12 In PHG, the coverage in A was higher than in B and C, in March, April, July and August, and it was higher in B than in A and C in May and June.
Among the three study sites, the highest (%) coverage of seagrass was recorded in PHG from March to June. But, its coverage was lower than IDG and KNG in July and August. TSC is shown in Figure 13. And TSMB (gdry.wtm-2) in PHG was lower than IDG and KNG. Leaves and sheaths weight were always lower than root + rhizome weight. The variations of the ratios of leaves, sheaths and roots + rhizomes were 1:1:4, 1:1:5, 1:1:10, 1:1:15, 1:2:7, 1:4:7, 1:5:8, 2:1:5, 2:1:7 and 3:2:17 at the three sites during the study period. Mean biomass (gdry.wtm-2) of leaves, sheaths and roots + rhizomes are represented in Figure 14.
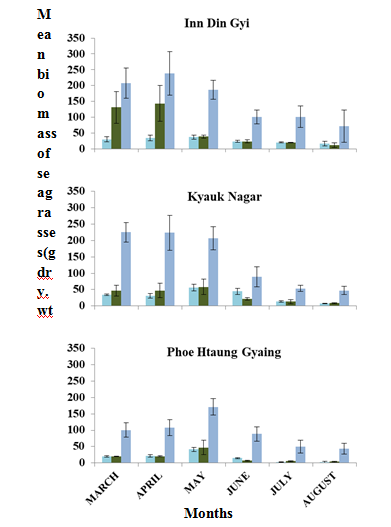
Figure 14 Mean biomass (gdry.wtm-2) of leaves, sheaths and roots + rhizomes in three study sites of Myanmar coast, from March to August, 2018.
In IDG, TSMB was higher than those in the transects A and C, in the transect B, in March and April; in the transect A, it was higher than those in the transects B and C, in May, and in the transect C was higher than those in the transects A and B, from June to August. In KNG, TSMB was higher than those of transect B and C from March to May in transect A; in transect B, it was higher than those of transects A and C, in June, and in transect C, it was higher than those in transect A and B, in July and August. In PHG, TSMB was higher than those of transect B and C in March, April and May, in transect A. But in transect B, it was higher than those in transect A and B, in June, July and August. Transect C values were always lower than those of the transect A and B during the study period (Figure 15). TSMB was generally higher in the dry season than in the monsoon season (Figure 16).
Although, a total of 11 species of seagrasses have been reported in Myanmar coast,4,5,21,25– 29 a total of 8 species were recorded in this study during the survey period, since H. beccarii,H. decipiens, H. ovalis were not found and Enhalusacoroides, growing in deep water of PHG was found outside of the transect lines which were exposed to air.
In IDG, a total of 6 species were recorded, namely Syringodiumisoetifolium, Cymodoceaserrulata, C. rotundata, Haloduleuninervis, Halophila major,Thalassiahemprichii. This last was the most dominant species during the study period. In KNG, also a total of 6 species were recorded, namely Syringodiumisoetifolium, Cymodoceaserrulata, C. rotundata, Haloduleuninervis, Thalassiahemprichii, Halophila major. Cymodoceaserrulata was the most dominant species during the study period. Soe-Htun et al.27 described the highest number of 10 species of seagrass, in PHG, whereas a total of 7 species were found in this survey in this site, including Cymodoceaserrulata, C. rotundata, Thalassiahemprichii, Haloduleuninervis, Enhalusacoroides, H. major and H. pinifolia. This last showed the most dominant species in PHG (Table 1). This discrepancy could be due to the wide extension of the Phoe Htaung Gyaing.
The highest biomass of seagrasses was observed in the IDG and KNG due to the presence of Thalassiahemprichii, and Cymodoceaserrulata. During the study period, Enhalusacoroides were found in calm water in the bay of PHG. Seagrass species revealed differences in distribution patterns: Thalassia was more dominant on the upper shore, whereas Halophila major, Haloduleuninervis and H.pinifolia were more dominant in mid shore. Cymodoceaserrulata and Enhalusacoroides were distributed in the deeper sites, due perhaps to the larger size of the plant, which was mostly submerged in all time.
Leaves and sheaths weight (above-ground) were always lower than roots + rhizomes weight (below-ground) because of large root and rhizome accumulation, which had not been removed or damaged. The above-ground part, however, might have been damaged and died off in dry season and also it could have been snapped off and washed up during the monsoon season. During the monsoon season, seagrasses seemed to have suffered from heavy wave actions and rainfall and turbidity by sediments more than during the dry season, thus decreasing both the above-ground and below-ground biomass. Such disturbance could reduce the coverage and biomass of seagrasses in three study sites.
In all the sites studied, TSC and TSMB were generally lower in monsoon season than in dry season. This could mainly be due to the strong wave action, sedimentation, rain fall, salinity variation, temperature and light reduction. In IDG, heavy sedimentation was in fact found in monsoon season. The variation of TSC along different transects was mainly due to the slightly changes of environmental parameters in deep water. In KNG, the coverage of seagrass of transect C was the higher in monsoon season than transects A and B because transect C was always submerged avoiding suffering from strong wave actions, compared to transects A and B. KNG possessed rocky shore and the waves were very strong in monsoon season. According to Soe-Htun et al.,27 the highest percent of seagrass meadows coverage was observed in PHG with 66.9%. In this study, among the 3 sites, the highest total coverage of seagrass was recorded in PHG with 75%. PHG resulted a valuable site and showed very wide and dense seagrass meadows, despite turbidity was very high. Although TSC of PHG was higher than other two sites from March to June, TSMB of PHG always was lower than other two sites due to the small species, such as Haloduleuninervis and H. pinifolia, dominant in PHG (Figures 13,16).
TSMB and TSC decreased in monsoon season with the lowering of the values of temperature, salinity and light (Figure 11). These parameters regulate seagrass biomass and coverage as well as seagrass growth (productivity). Decreased seagrass biomass and coverage is likely due to the strong wave action, rain fall, sedimentation, salinity variation, temperature and light reduction in Southern Rakhine Coastal Area (Shwe Thaung Yan).
The sea grass meadows along the Rakhine Coastal Region are known to serve as important feeding grounds for the sea cow, Dugong dugon, and five species of seaturtles which is listed as Vulnerable on the IUCN Red list.30 Spatial cover of seagrasses was very healthy condition in Shwe Thaung Yan coastal area. The three study sites were feeding ground for endangered species dugong (Dugong dugon) and sea turtles (Dermochelyscoriacea, Eretmochelysimbricata, Lepidochelysolivacea, Carettacaretta, Cheloniamydas). Shwe Thaung Yan coastal area is very suitable to demarcate Marine Protective Area (MPA) because it possesses very important natural resource (mangrove forest, seagrass beds and coral reef). Many factors can lead to seagrass loss in Shwe Thaung Yan, primarily turbidity due to sewage discharges, storm-water, dredging, land reclamation and changes of environmental parameters.
It is essential to monitor continuously the growth rate of seagrasses, seasonal changes effect on the seagrass beds, environmental parameters effect on seagrass to more understand ecology of seagrass, to improve coastal zone management.
We would like to express our gratitude to Dr Si Si Hla Buu, Rector, Dr. Ni Lar Myint, Pro-Rector and Dr. Than Htoon, Pro-Rector, Pathein University for their supports to undertake this research work. We are profoundly grateful to Dr. Htay Aung, Associate Professor (Retired), Department of Marine Science, Pathein University, for the continuous support and encouragement during this work. We are especially indebted to Dr. Tint Lwin, Dr. Sa Aung Myo Htay, Dr. Khin Maung Naing, U Thant Zin, Dr. Thu Thu Min and all staffs of Department of Marine Science, Pathein University, for their helpful assistance during the period of this studies. Last but not the least, the first author, Thu Rein would like to deepest thanks to his parents and sisters, supporting him financially and spiritually throughout his life.
The author declares that there are no conflicts of interest.

©2019 Thu-Rein,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.