Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 2
Programa de Pós-Graduação em Geodinâmica e Geofísica (PPGG), Universidade Federal do Rio Grande do Norte (UFRN), Brazil
Correspondence: Patrícia P. B. Eichler, Programa de Pós-Graduação em Geodinâmica e Geofísica (PPGG), Universidade Federal do Rio Grande do Norte (UFRN), Campus Universitário, Lagoa Nova, 59072-970, Natal, RN, Brazil
Received: July 25, 2024 | Published: August 15, 2024
Citation: Eichler PPB, Vital H, Gomes MP. Resilience of reefal foraminifera to anthropogenic influences from reef areas (Pirangi, Maracajaú, and Açu) in Rio Grande do Norte (RN, Brazil). J Aquac Mar Biol. 2024;13(2):85-95. DOI: 10.15406/jamb.2024.13.00401
We have studied seven symbiont-bearing foraminiferal species to evaluate the condition of sedimentary reef areas (Pirangi, Maracajaú, and Açu) in Rio Grande do Norte (RN, Brazil). The species are: Amphisorus hemprichii, Amphistegina gibbosa, Archaias angulatus, Borelis schlumbergeri, Heterostegina antillarum, Peneroplis carinatus, and Laevipeneroplis proteus. This paper focuses on the quantitative distributions of the first two species in Maracajaú and Pirangi, considering micro-habitat variation, shelf zonation, and anthropogenic disturbances. Amphistegina gibbosa, which is more abundant than A. hemprichii, is prevalent in coral or coral-rubble substrates, whereas Amphisorus hemprichii is better represented in seagrass habitats. Our dataset from Pirangi and Maracajaú is based on 123 samples collected over three years through diver collection, grab samples, and underwater videographic surveys. Compared to other Brazilian reefs, species diversity at Pirangi is low, and the community is impoverished near tourism sites. These areas, especially Pirangi, may also be affected by pollution from domestic sewage and industrial waste. In Maracajaú, the community appears healthier, except in sites heavily impacted by tourism. Data from 84 foraminiferal samples from Açu show well-preserved symbiont-bearing foraminifera (SBF), indicating good water quality, with microhabitat variations likely due to natural factors. A quantitative examination of the foraminiferal species revealed the presence of a Caribbean-type reef community, including Amphisorus hemprichii, Amphistegina gibbosa, Archaias angulatus, Heterostegina antillarum, Homotrema rubra, Peneroplis carinatus, and Laevipeneroplis proteus. Additionally, among 65 species, living Buccella peruviana were found with a very restricted distribution in organic-rich sediments associated with upwelling wind events, highlighting areas of cold water upwelling on the outer shelf. The spatial distribution of this species indicates that cold waters are bringing nutrients through tidal currents perpendicular to the shelf edge, likely using canyons and valleys to transport nutrients and cold water masses into the partially filled incised valley of the ancient Açu River. Changes in diversity appear to be primarily induced by anthropogenic influences on the inner shelf rather than the outer shelf.
Keywords: symbiont-bearing protest, hermatypic marine
Foraminifera are a diverse group of amoeboid protists characterized by their quite complex shell structures, known as tests. These microorganisms are found in marine environments worldwide and are integral to the marine ecosystem due to their roles in the carbon and nitrogen cycles and as bio indicators of environmental conditions.1,2 Foraminiferal assemblages can provide valuable information about past and present environmental conditions, making them essential for studies on climate change, pollution, and habitat health. They are commonly used as bioindicators in marine environments due to their sensitivity to environmental changes. They play a crucial role in assessing the health of coral reef ecosystems and understanding past climatic conditions.3,4
The Rio Grande do Norte shelf in Brazil, featuring notable reef areas such as Pirangi, Maracajaú, and the Açu Incised Valley, offers a diverse range of habitats for foraminiferal species. The coral reef systems in these areas are vital for coastal protection, biodiversity, and local economies dependent on tourism and fishing. However, these ecosystems are increasingly threatened by anthropogenic activities such as pollution, overfishing, and climate change.5,6
To assess the condition of these sedimentary reef areas, surface sediment samples were collected from these three key locations: Pirangi, Maracajaú, and the Açu Incised Valley. We have considered various factors such as micro-habitat variation, shelf zonation, and anthropogenic disturbances, providing a comprehensive view of the current state of these reef ecosystems. In Pirangi and Maracajaú, we focused on the quantitative distributions of two main symbiont-bearing foraminifera species: Amphisorus hemprichii and Amphistegina gibbosa because we already know that the differences in species distributions between Pirangi and Maracajaú underscore the effects of tourism and pollution, with areas like Pirangi showing lower diversity and signs of degradation near tourist sites.7 Amphistegina gibbosa is often found in coral rubble substrates, while Amphisorus hemprichii prefers seagrass habitats.8 We have examined these two species in 95 samples because they are particularly indicative of reef health and environmental stability.9 In the Açu Incised Valley, a broader approach was taken by examining the entire foraminiferal assemblage from 84 stations. This area, characterized by its complex geological and hydrodynamic conditions, offers a unique opportunity to study the effects of nutrient upwelling and sediment transport on foraminiferal communities.10 The presence of well-preserved symbiont-bearing foraminifera in Açu indicates good water quality and minimal anthropogenic impact.11
Our findings highlight the importance of foraminifera as indicators of reef health and environmental changes. By comparing the assemblages across these diverse habitats, we aim to identify patterns and drivers of ecological change, contributing to the conservation and management of these critical marine environments. Also, we emphasize the need for effective environmental management and protection strategies to preserve these vital ecosystems. By monitoring foraminiferal communities, we can gain valuable insights into the health of coral reefs and the impacts of human activities, informing conservation efforts and policy decisions.8
Foraminifera constitute an inexpensive and easily-handled proxy for monitoring coastal environmental stressors, including elevated temperatures, acidification, and influx of pollutants. Shifts in local foraminiferal assemblages associated with coral reefs may help differentiate a long-term reef decline caused by deteriorating water quality from a temporary decline associated with episodic mortality events.12 These shelled microorganisms, because of their high numerical abundance and species diversity, are dependable indicators of environmental perturbations,13,14 and thus, reef foraminifera can be utilized as “bio indicators of coral reef health”.15
Here we show the environmental controls on the distribution of two symbiont-bearing foraminfera in three coral-reef areas on the northeastern Brazilian shelf (Açu, Maracajaú, and Pirangi, Figure 1, retired from Eichler and Barker.16 Figures 2, 3, and 4 are detailed locations of sampling in Pirangi, Maracajaú and Açu and it was modified from Eichler et al.8
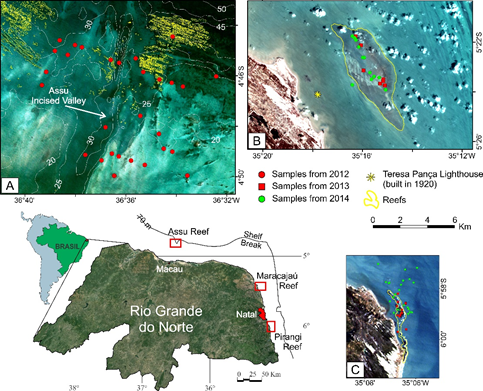
Figure 1 Sampling sites for foraminiferal species in three coral reef areas, Rio Grande do Norte, Brazil: Açu, Maracajaú, Pirangi16 from page 23 (Fig. 1.15).

Figure 2 A. Station locations for surface-sediment Pirangi samples (2013). B. Pirangi samples (2014).
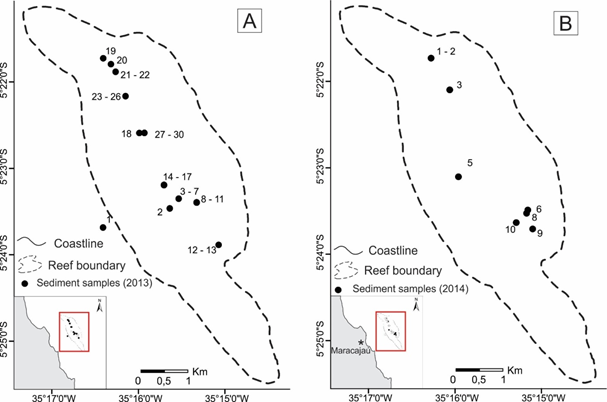
Figure 3 A. Station locations for surface-sediment Maracajaú samples (2013). B. Maracajaú samples (2014).
Regional setting
The continental shelf of Rio Grande do Norte State (4.8°–7.5°S; 34.8°–37.2°W) is a part of the southwestern equatorial Atlantic Margin (Fig. 1; Vital et al., 2010). This shelf is very shallow (shelf break at 70 m water depth), narrow (<40 km wide), with gentle gradient (<1°), and smooth relief.17,18 Sedimentary and geomorphological characteristics permit the separation of the shelf into inner (<15 m), middle (15–25 m), and outer (25–70 m) segments.19 Subaqueous dunes and shallow, isolated sand bodies are present in the inner shelf; submerged beachrock chains bound the middle and outer shelves, and two incised valleys cut the shelf from the coast to the shelf break.20–24
Mixed carbonate-siliciclastic sediments cover the modern shelf. Siliciclastic sediments predominate on the inner shelf; the carbonate component and grain-size increase toward the middle and outer shelves.22,23 In the northern part of the shelf, a coral reef field was recently discovered and named Açu Reefs,23 a subject of the present study. These reefs occur at depths below 20 m in the outer shelf, in a narrow (6-km wide) band with the steepest gradient (1:250). Coarse, carbonate-rich sediments are predominant in this area. The bio constructions attain heights up to 15 m as mounds, knolls, and banks that are isolated or aligned parallel to the shelf break. Gomes et al.23 believe that these reefs are related to an ancient fringing coral reef, now detached from the coast. The fossil reefs contain hermatypic corals. Preliminary observations also reveal living corals, sponges, and green algae in this area (e.g. Montastraea cavernosa, Porites astreoides, Siderastrea stellata, Scopalina ruetzleri, Callyspongia vaginalis, Ectyoplasia ferox, Ircinia sp., Spirastrella sp., and Aiolochroia sp.,23 and some of them are illustrated in Figure 5.
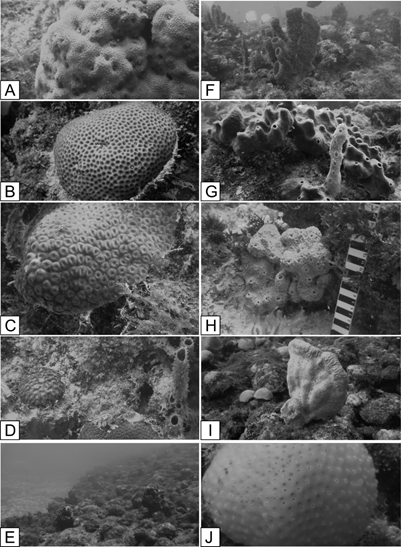
Figure 5 Açu Reefs, underwater photos of sponges and reef corals. a) Ectyoplasia ferox b) Siderastrea stellata c) Montastraea cavernosa d) Porites astreoides e) Scopalina ruetzleri f, g) Callyspongia vaginalis h) Spirastrella sp i) Aiolochroia sp j) Ircinia sp.
East of the Natal-Maracajaú coast (Figure 1), the reef fields of Maracajaú and Pirangi occur in the nearshore and inshore environments, respectively. The small reefs of Pirangi are about 25 km south of Natal in less than 5 m of water (Figure 2). The Maracajaú reef is part of a chain (Sioba, Cação, Rio do Fogo, and Maracajaú Reefs (Figure 3). It lies at about 5 km offshore at depths of 3 to 5 m, and consists of knolls and pinnacles that reach up to 6 m in height, and may be partially exposed at low tide.21 The bio construction is mainly composed of Siderastrea stellata (80%), but Agaricia agaricites, Agaricia fragilis, Meandrina brasiliensis, Millepora alcicornis, Mussismilia harttii, Porites astreoides, Porites branneri and Favia gravida are also present. Montastraea cavernosa occurs at greater depths.21,25
The climate varies from dry tropical and semi-arid in the area north of Macau to tropical and humid east of Natal. Temperatures vary between 28ºC (summer of December-January) and 18ºC (winter of June-August). Figure 4 shows location of sampling and temperature when sampling of sediment sampling was done in the Açu Incised Valley. The salinity is higher than 37.0 PSU in both areas.26 The eastern area has a wave-dominated coast with active sea cliffs carved into tablelands, while the northern area is a mixed-energy complex of tide-modified coast.27 A semidiurnal, meso-tidal regime dominates the two shelf areas, with a maximum spring-tide range of 3.3 m and a minimum neap-tide range of 1.2 m in the north, and corresponding values of 2.2 m and 1.0 m in the east.
Anthropogenic activities in the three reefal areas
Pirangi
The “Marina Badauê Boat Tours” and other tourist activities take tourists to explore the Natural Pools of Pirangi, 800 meters from the coast. They leave from Pirangi beach three times a day year-round; tour itinerary lasts an average of two hours, with a stop for swimming and diving in the natural pools. At low tide, the coral reefs are exposed, and small pools are created where it is possible to observe small, colorful fish without the use of a mask. The tour company promotes walking on the reefs and rents sandals for that purpose.28
While easily reached by tourists, its close proximity to the coast also exposes the Pirangi reefs to domestic and industrial waste carried by the Pirangi Watershed.29
Maracajaú
Maracajaú Beach, 60 km north of Natal, is famous for the coral reef communities 7 km from the coast. During the low tide, the area transforms into a natural pool of crystalline water, the depth varying between 1.0 and 3.0 m, with a beautiful and rich marine fauna and flora. It is considered one of the ten best places in Brazil for snorkeling and scuba diving for its natural conditions. Tourist boats at the Maracajau reefal area; the visitors are swimming and snorkeling rather than walking on the reef, in water depths greater than at Pirangi. The Maracajaú reefs belong to a conservation unit called the "Environmental Protection Area of Coral Reefs” (APARC), created in 2001. According to Araújo and Amaral,30 APARC and the adjacent continental shelf are currently experiencing an increase in environmental degradation due to overfishing, coastal occupation and increasing tourism.
Açú
Located on the outer shelf at depths of 20 to 60 m, the mesophotic Açu reefs may be less susceptible to anthropogenic influences than shallow reefs.23 Oil refineries near Macau and petroleum production in the area, however, do have the potential to affect the reefs.31,32 Overfishing also remains a threat to the marine ecosystems of Rio Grande do Norte.30 Unlike Pirangi and Maracajaú, Açu does not have regular tourist excursions for scuba diving and snorkelling.
Sampling
Surface sediments were collected by deploying a Multi core samples from the boat, and by scuba divers at 55 stations at Pirangi, 40 stations at Maracajaú, and 84 stations at the Açu in water depths from 40 and 65 m (Figure 2, 3, 4). Scuba divers to evaluate the reef macrobenthic communities, to collect sediment samples for granulometry and for foraminiferal studies, and to conducted underwater video-graphic and photographic surveys. For Maracajaú and Pirangi, sampling was done in winter (July 2012, June 2013, and July 2014). For Açu samples were collected in July 2012, 2015 and March 2016). Water temperature, salinity, and dissolved oxygen were recorded in the field a probe then we have plotted Açu sampling map and temperature.
Sediment and biological samples were sub-sampled with a spatula, and about 10 cm3 of sediment were removed, preferentially from the top layer (<1 cm).
Foraminiferal analysis
The material collected was preserved in an ethanol-Rose Bengal solution, in order to stain the cytoplasm of live foraminifera. Processing of sediments followed standard procedures. A fixed volume of 10 cm3 of sediment was washed through a 0.063 mm sieve. After drying, samples were partitioned by a micro splitter into subsamples that contained about 100 foraminiferal specimens. All specimens were counted when the number of foraminifers in a sample was less than 100 in the entire sample. Species identification and counting of dry specimens were done under an optical microscope. Because only a few tests were stained by Rose Bengal, all our data are based on total foraminiferal counts. Species of benthic foraminifera were picked from sieved and washed residues (>63 µm), and identified under a stereo binocular microscope; digital images were taken with a Nikon Coolpix 995 camera. For some specimens, scanning electron micrographs were obtained to resolve ambiguous identifications.
Sediment analysis
Sediment grain size was analyzed, using 30 g of each sample. Hydrochloric acid (10%) was added to this material until the bio detrital calcium carbonate was eliminated. The remaining material was dried at 60°C, weighed, and then washed through a 0.063-mm sieve. The coarser fraction was dried, weighed, and sieved at half phi (f) intervals between 4 and 0.063 mm. The fraction finer than 0.063 mm was put into suspension in 1000 ml of distilled water, to which 1 gram of Na4P2O7 was added to help deflocculation. Suguio’s method33 was then used for pipette analysis of the silt-clay fraction.
Organic matter
For determining organic matter content in the marine sediment samples we have used hydrogen peroxide (H2O2) digestion. The procedure involves collecting and air-drying sediment samples, which are then crushed and homogenized into a fine powder. Approximately 1-5 grams of the dried sediment is accurately weighed and placed into a clean glass beaker. To initiate the reaction, 10-20 mL of 30% H2O2 per gram of sediment is added slowly to the beaker to avoid overflow. The beaker is then heated on a hot plate or in a water bath at 60-80°C to accelerate the reaction, although the process can also be performed at room temperature for a longer duration. The reaction is monitored by observing the bubbling and froth, indicative of H2O2 reacting with the organic matter. Additional H2O2 is added if the reaction slows until bubbling ceases. The pH of the solution is checked periodically and adjusted to neutral (around pH 7) using a neutralizing agent such as sodium bicarbonate if it falls below 4. After the reaction is complete, the mixture is allowed to cool to room temperature. If the mixture remains turbid, it is filtered to separate the residual inorganic material. The filtered residue is then dried in an oven at 100-105°C until a constant weight is achieved, ensuring all moisture is removed. The dried residue is accurately weighed, and the organic matter content is calculated using the weight loss method. The percentage of organic matter is determined by comparing the initial weight of the sediment to the weight of the residue after H2O2 digestion.
Statistical analysis
Multivariate analysis using the BEST method34 was implemented with PRIMER 6 (Plymouth Routines in Multivariate Ecological Research, PRIMER-E Ltd., Plymouth, UK). This procedure attempts to find the best match between biotic (e.g., foraminiferal species) and abiotic (e.g., grain size) data sets.
Coral reef substratum
Our findings based on quantitative data of 95 samples from Pirangi and Maracajaú and 84 samples of Açu point out that Pirangi bio constructions occur as patches composed by sedimentary sponge and algae as the main structure of support to the coral communities and other living species. They attach themselves to something solid for their survival. The sedimentary reef in Pirangi is made of dead sponge and algae, and it is not a sedimentary rock composed of skeletal of Coral, Foraminifera and Mollusks like the ones found in Southern Australia. We have observed that the reef in Pirangi occurs as patches constructed primarily of sedimentary sponge and algae instead of the remains of dead coral, foraminifera, and mollusks. The living coral communities and other invertebrates have attached to this hard substrate.
Symbiont-bearing foraminiferal species
The foraminiferal assemblage of Rio Grande do Norte includes seven species with algal endosymbionts (“symbiont-bearing-foraminifera” or SBF in this text). These SBF are: Amphistegina gibbosa d’Orbigny (Figure 6), Amphisorus hemprichii Ehrenberg (Figure 7), Archaias angulatus (Fichtel & Moll), Borelis schlumbergeri (Reichel), Heterostegina antillarum d’Orbigny, Peneroplis carinatus d’Orbigny, and Laevipeneroplis proteus (d’Orbigny) (Figure 8). Such species are known to flourish in nutrient-poor waters of coral reefs. The affinity of the assemblage is distinctly Caribbean.
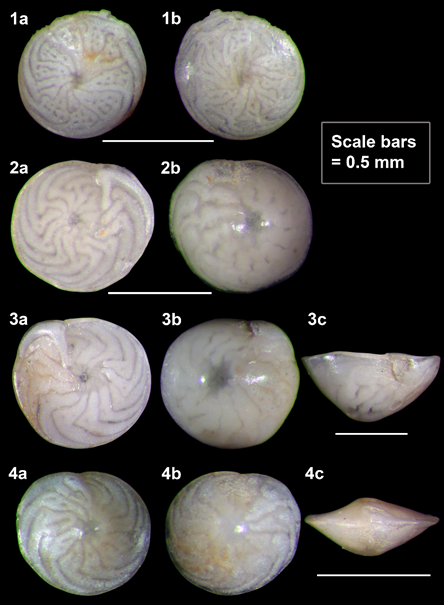
Figure 6 Four individuals of Amphistegina gibbosa, Rio Grande do Norte, digital photomicrographs. a, dorsal view; b, ventral view; c, edge view. 1, 4, biconvex (lenticular) form; 2, 3, planoconvex form. A colorful reproduction of the figure 1 from Eichler et al.8
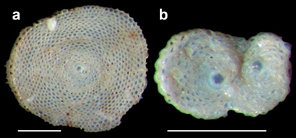
Figure 7 Amphisorus hemprichii, Rio Grande do Norte; a, normal form; b, twinned form. Scale bar = 0.5 mm. Retired from Eichler et al 20198 (Figure 2 from the paper).

Figure 8 Five other symbiont-bearing foraminifers, Rio Grande do Norte. a, Archaias angulatus; b, Borelis schlumbergeri; c, Heterostegina antillarum; d, Peneroplis carinatus; e, Laevipeneroplis proteus. Scale bars = 0.5 mm in a, c, d, and e; 0.1 mm in b.
The best-known SBF genus of coral reefs and other shallow, tropical carbonate banks or hard ground is Amphistegina, and A. gibbosa is the most conspicuous member of the SBF group in our samples. Its distribution may be wider than what is reported in the literature, because of the confusion created by its morphological similarity with the widespread A. lessonii. However, the two taxa can be distinguished by (a) the abundance and complexity of chambers, (b) the extent of the papillate area, and (c) the relative convexity of the dorsal and ventral sides, notwithstanding intraspecific morphological variations;35 in our samples, large specimens of A. gibbosa are typically planoconvex (see Figure 6, Illustration 3c), but biconvex specimens also occur (Figure 6, 4c). In the Caribbean Sea and Gulf of Mexico, A. gibbosa is apparently the only species of Amphistegina.35,36 Amphisorus hemprichii (Figure 7) is the only flat, discoid species among the Rio Grande do Norte SBF. Varieties of endosymbionts are hosted by SBF species, e.g., diatoms by Amphistegina spp. and dinoflagellates by Amphisorus hemprichii.37
Comparison of abundances from Pirangi and Maracajaú
Data from 95 samples were compared in terms of absolute abundances of two symbiont-bearing foraminiferal species, Amphistegina gibbosa and Amphisorus hemprichii (Table 1). The three studied reefal areas (Pirangi and Maracajaú, inner shelf; Açu, outer shelf) are subject to anthropogenic impacts related to tourist activities, domestic waste, the petroleum industry, and fishing. Type of pollution, sample coverage, and sampling years are given in Table 2 for each reef.
Table 1 Absolute numbers of Amphistegina gibbosa and Amphisorus hemprichii from Pirangi and Maracajau (2013, 2014).8
|
Reef area |
Pollution source |
Number of samples |
Sampling year |
|
Açu |
Petroleum industry/ Fishing |
84 |
2012, 2015 and 2016 |
|
Pirangi |
Domestic waste/ Tourist activities |
55 |
2013/2014 |
|
Maracajaú |
Tourist activities |
40 |
2013/2014 |
Table 2 Sampling areas and known kinds of pollution
At the Pirangi site in 2013, Amphistegina gibbosa was abundant everywhere except at station 27 (Table 1), which is closest to the location where people step on the reef. Relatively high numbers of A. gibbosa were found at stations 15, 22, 23, 24, and 25, all stations in the outer reef with the exception of 15, the closest station to the Pium River. Amphisorus hemprichii occurred at only six stations: 15, 16, 17, 18, 20, and 27. In 2014, Amphistegina gibbosa occurred with high numbers at stations 3, 6, 7, 9, 16, and 24, all located in the inner part of the reef. Relatively high numbers of Amphisorus hemprichii were found at widely scattered stations (1 to 6, 8 to 12, 15, 20, and 22). Living individuals of A. hemprichii were found only in sea grass habitats.
In 2013, samples from the Maracajaú inner shelf contained Amphistegina gibbosa in just over 50% of the samples, with higher densities at stations 1, 2, 12, and 13 (Table 1) in the southern part of the reef, and at station 19 in the northern part; Amphisorus hemprichii occurred at stations 1, 6, 7, 15, 17, 21, and 23 to 30. In 2014, Amphistegina gibbosa was present at all stations, with higher abundances at 1, 4, 5, 8, 9, and 10. Amphisorus hemprichii was present only at stations 1, 2, 5 and 6. Depletion of the foraminiferal community is obvious in the part of the Pirangi reef that is impacted by tourist activities. In the survey of 2014, when we sought to collect from more diverse stations in Pirangi, both Amphisorus and Amphistegina seemed more widespread. The best development of symbiont-bearing foraminifera (seven species, Figures 6–8) was found in the outer shelf part of Açu Reefs (deeper than 25 m).
The BEST analyses (Table 3) performed on sediment data from Pirangi and Maracajaú (Tables 4 and 5) revealed percentage coarse fraction and percentage sand as the variables that best correlate with the presence/absence of Amphistegina gibbosa and Amphisorus hemprichii (Table 1, Figure 9). Water depth in these two study areas has less effect on the distribution of foraminifera. In general, the low abundance of symbiont-bearing taxa and the dominance of smaller taxa, including stress-tolerant species, indicate less-than-optimum conditions for reef-growth in the two study areas. The foraminiferal community is particularly depauperate around the reefs of Pirangi. In the more extensive patch reef system of Maracajaú, this community is apparently healthier, except where impacted by tourist activities that have physically damaged the reef substrate.
|
No. Vars |
Corr. selections |
|
4 |
0,428 2;4-6 |
|
5 |
0,426 1;2;4-6 |
|
4 |
0,426 1;2;5;6 |
|
3 |
0,424 2;4;6 |
|
4 |
0,424 1;2;4;6 |
|
3 |
0,423 1;2;5 |
|
3 |
0,422 2;5;6 |
|
3 |
0,422 1;2;6 |
|
2 |
0,421 2;5 |
|
4 |
0,419 1;2;4;5 |
Table 3 BEST results (Biota and Environment matching) from Maracajau 2014. Rank correlation method: Spearman Method: BIOENV using analyse between samples (Resemblance measure: D1 Euclidean distance). Variables: 1 Coarse (%) 2 Sand (%) 3 Silt (%) 4 Clay (%) 5 TOM (%) 6 Caco3 (%)
|
Pirangi 2013 |
Latitude |
Longitude |
Depth (m) |
Coarse (%) |
Sand (%) |
Silt (%) |
Clay (%) |
TOM (%) |
CaCO3 (%) |
|
1 |
5°57'11" |
35°05'55.8" |
12.0 |
27.8 |
70.8 |
1.1 |
0.3 |
0.0 |
32.8 |
|
2 |
5°57'37.6" |
35°06'13.5" |
9.5 |
2.1 |
97.8 |
0.1 |
0.0 |
0.8 |
3.7 |
|
3 |
5°57'45.8" |
35°06'40" |
12.0 |
23.6 |
56.7 |
11.0 |
8.7 |
3.2 |
58.7 |
|
7 |
5°58'01.5" |
35°07'27.5" |
5.4 |
0.0 |
65.2 |
17.0 |
17.9 |
9.1 |
39.4 |
|
8 |
5°57'41" |
35°06'28.7" |
13.0 |
3.8 |
96.2 |
0.0 |
0.0 |
1.0 |
19.4 |
|
9 |
5°57'50.8" |
35°06'-33.6" |
13.5 |
16.9 |
82.9 |
0.2 |
0.0 |
2.1 |
44.8 |
|
10 |
5°58'02.9" |
35°06'37.9" |
10.0 |
0.6 |
99.4 |
0.0 |
0.0 |
1.3 |
73.9 |
|
11 |
5°58'11.9" |
35°06'41.8" |
11.0 |
11.6 |
88.2 |
0.2 |
0.0 |
1.6 |
81.0 |
|
15 |
5°58'29.8" |
35°07'00.2" |
4.6 |
0.0 |
98.7 |
1.3 |
0.0 |
4.9 |
56.1 |
|
17 |
5°58'57.0" |
35°06'32.0" |
0.7 |
44.5 |
55.0 |
0.5 |
0.0 |
2.7 |
81.1 |
|
18 |
5°58'57.0" |
35°06'32.0" |
1.0 |
17.7 |
81.7 |
0.7 |
0.0 |
2.8 |
84.0 |
|
22 |
5°58''44.5" |
35°06'18.1" |
11.0 |
0.1 |
99.4 |
0.5 |
0.0 |
14.7 |
45.2 |
|
26 |
5°58'59.6" |
35°06'17.1" |
4.0 |
9.9 |
89.1 |
0.9 |
0.0 |
3.0 |
32.3 |
|
27 |
5°58'50.3" |
35°06'34.9" |
2.5 |
40.5 |
57.6 |
1.9 |
0.0 |
3.4 |
88.1 |
|
28 |
5°58'50.3" |
35°06'34.9" |
2.5 |
16.0 |
84.0 |
0.0 |
0.0 |
2.9 |
98.4 |
|
29 |
5°58'49.4" |
35°06'41.4" |
3.5 |
4.4 |
60.4 |
14.6 |
20.6 |
4.7 |
50.6 |
|
30 |
5°58'57.2" |
35°06'51.0" |
3.5 |
0.0 |
99.1 |
1.0 |
0.0 |
2.1 |
23.6 |
Table 4 Pirangi stations and sediment components (Latitude, longitude, depth, coarse sand, sand, silt and clay). Data previously published in Eichler and Barker (2020) and Eichler et al. (2019b)
|
Maracajaú 2013 |
Latitude |
Longitude |
Depth (m) |
Coarse (%) |
Sand (%) |
Silt (%) |
Clay (%) |
TOM (%) |
CaCO3 (%) |
|
1 |
05° 23' 42.4" |
35° 16' 24.1" |
3.5 |
1.7 |
97.7 |
0.5 |
0.1 |
2.3 |
93.9 |
|
2 |
05° 23' 29.2" |
35° 15' 37.7" |
4.0 |
0.0 |
99.9 |
0.1 |
0.0 |
2.7 |
98.6 |
|
3 |
05° 23' 22.5" |
35° 15' 31.6" |
2.5 |
0.4 |
70.3 |
15.1 |
14.2 |
4.8 |
87.3 |
|
4 |
05° 23' 22.5" |
35° 15' 31.6" |
3.5 |
0.1 |
61.6 |
15.8 |
22.6 |
6.1 |
85.0 |
|
5 |
05° 23' 22.5" |
35° 15' 31.6" |
3.5 |
0.0 |
32.8 |
34.0 |
33.2 |
15.0 |
76.4 |
|
6 |
05° 23' 22.5" |
35° 15' 31.6" |
3.5 |
2.1 |
44.6 |
22.7 |
30.7 |
6.4 |
84.9 |
|
7 |
05° 23' 22.5" |
35° 15' 31.6" |
3.5 |
1.1 |
37.6 |
33.0 |
28.4 |
4.6 |
86.6 |
|
8 |
05° 23' 25.2" |
35° 15' 19.0" |
2.5 |
1.6 |
96.1 |
1.9 |
0.3 |
3.2 |
96.2 |
|
9 |
05° 23' 25.2" |
35° 15' 19.0" |
2.5 |
4.2 |
60.2 |
19.4 |
16.2 |
8.2 |
88.0 |
|
10 |
05° 23' 25.2" |
35° 15' 19.0" |
2.5 |
4.8 |
87.1 |
6.5 |
1.6 |
3.8 |
97.9 |
|
11 |
05° 23' 25.2" |
35° 15' 19.0" |
2.5 |
0.1 |
79.1 |
5.5 |
15.3 |
2.6 |
97.1 |
|
12 |
05° 23' 54.8" |
35° 15' 03.9" |
4.0 |
2.9 |
96.8 |
0.3 |
0.1 |
1.0 |
99.1 |
|
13 |
05° 23' 54.8" |
35° 15' 03.9" |
3.2 |
1.1 |
99.0 |
0.0 |
0.0 |
3.3 |
97.7 |
|
14 |
05° 23' 13.0" |
35° 15' 41.6" |
2.1 |
2.7 |
72.8 |
10.9 |
13.5 |
4.5 |
76.6 |
|
15 |
05° 23' 13.0" |
35° 15' 41.6" |
3.7 |
1.9 |
51.2 |
29.6 |
17.3 |
4.7 |
92.5 |
|
17 |
05° 23' 13.0" |
35° 15' 41.6" |
2.5 |
20.2 |
36.9 |
35.7 |
7.2 |
7.7 |
63.7 |
|
18 |
05° 22' 36.7" |
35° 15' 58.7" |
2.5 |
2.0 |
75.7 |
15.1 |
7.3 |
3.5 |
97.2 |
|
19 |
05° 21' 44.9" |
35° 16' 23.5" |
4.0 |
8.4 |
91.1 |
0.4 |
0.1 |
3.4 |
99.4 |
|
20 |
05° 21' 48.9" |
35° 16 18.2" |
4.0 |
10.9 |
88.0 |
0.9 |
0.2 |
2.4 |
97.7 |
|
21 |
05° 21' 54.2" |
35° 16' 15.0" |
5.0 |
7.3 |
92.7 |
0.0 |
0.0 |
2.9 |
97.5 |
|
22 |
05° 21' 54.2" |
35° 16' 15.0" |
5.4 |
8.1 |
91.2 |
0.6 |
0.1 |
2.4 |
90.8 |
|
23 |
05° 22' 11.2" |
35° 16' 8.2" |
3.9 |
0.7 |
28.9 |
35.8 |
34.7 |
7.3 |
79.1 |
|
24 |
05° 22' 11.2" |
35° 16' 8.2" |
3.9 |
15.8 |
83.0 |
1.1 |
0.2 |
4.3 |
98.5 |
|
25 |
05° 22' 11.2" |
35° 16' 8.2" |
3.9 |
6.8 |
84.3 |
7.1 |
1.7 |
3.9 |
75.6 |
|
27 |
05° 22' 36.8" |
35° 15' 55.1" |
3.5 |
1.0 |
35.0 |
31.5 |
32.4 |
6.4 |
82.4 |
|
28 |
05° 22' 36.8" |
35° 15' 55.1" |
3.5 |
1.8 |
49.7 |
22.2 |
26.3 |
4.9 |
88.4 |
Table 5 Maracajaú stations and sediment components (Latitude, longitude, depth, coarse sand, sand, silt and clay). Data previously published in Eichler and Barker (2020) and Eichler et al. (2019b)
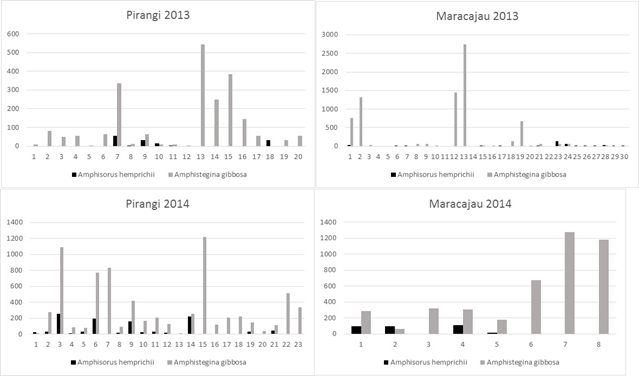
Figure 9 Absolute numbers of Amphistegina gibbosa and Amphisourus hemprichii at Pirangi and Maracajaú sampling stations (2013, 2014).
A quantitative examination of the foraminiferal species from the 84 core tops from Açu (Table 6) showed the presence of the following indicator species Amphisorus hemprichii, Amphistegina gibbosa, Archaias angulatus, Bolivina striatula, Buccella peruviana, Elphidium articulatum, Heterostegina antillarum, Laevipeneroplis proteus, Peneroplis carinatus, Peneroplis sp., Pyrgo sp., Pseudononion atlanticum, Quiqueloculina lamarckiana, Quiqueloculina patagonica, and Textularia earlandi.
Table 6 Absolute number of Foraminifera species in 85 samples.
Quinqueloculina lamarckiana, a calcareous robust form tolerant to high hydrodynamics, is the most dominant species, followed by Amphistegina gibbosa, Archaias angulatus, and Peneroplis carinatus. These species, along with Amphisorus hemprichii, Heterostegina antillarum, Laevipeneroplis proteus, and Peneroplis sp., are typical reefal forms found in carbonate environments.30 Textularia earlandi, a foraminifera that agglutinates sediment particles, inhabits sediments with gravel.1 There are also species tolerant to high organic matter levels such as Bolivina striatula, Pseudononion atlanticum, Quinqueloculina patagonica, and Elphidium articulatum, and species thriving in oxygenated waters like Hanzawaia boueana4 and the warm-water indicator Pyrgo sp.
Buccella peruviana, a cold-water species,38 occurs in stations 2, 10, 14, 17, 18, 19, 21, 23, 25, 28, 35, 39, 40, 43, 44, 45, 47, 50, 51, 56, 57, 58, 60, 61, 63, 66, 67, 68, 70, 73, 74, 79, 90, 91, 101, 103, 115, 116, and 140. The presence of this species in deeper parts of the study area, as well as in shallower areas, indicates the upwelling and penetration of cold waters into submerged reefal canyons. The presence of B. peruviana in shallower samples is indicative of colder waters reaching depths from 19.5 to 40 m, providing conditions for this species to proliferate.8
Archaias angulatus competes with Amphistegina gibbosa for high organic matter, CaCO3, gravel, fine sand, very fine sand, and silt. However, A. gibbosa proliferates in more silt, while A. angulatus prefers medium to very fine sand. Amphisorus hemprichii shows a similar preference to A. angulatus for medium to very fine sand, while Peneroplis carinatus shares the same preference for silt as Amphistegina gibbosa. The distribution of Textularia earlandi and Pseudononion atlanticum shows a preference for environments with low CaCO3 and low silt. Textularia earlandi, which agglutinates sediment particles, also correlates well with gravel.1 Amphisorus hemprichii and Pyrgo spp. are co-occurring, competing with Quinqueloculina patagonica and Textularia earlandi. Figure 10 shows similar patterns of Foraminiferal species (Amphistegina gibbosa, Peneroplis carinatus, Buccella peruviana, Peneroplis sp., Quinqueloculina lamarckiana, Archaias angulatus) distribution in the Açu area. Comparing data from Figure 10 and Table 6, Archaias angulatus and Amphisorus hemprichii prefer medium to very fine sand, Amphistegina gibbosa and Peneroplis carinatus proliferate in more silt, and Buccella peruviana and Quinqueloculina lamarckiana prefer high organic matter, carbonate, gravel, and silt. Archaias angulatus competes with Amphistegina gibbosa (Figure 11), with both displaying a preference for high organic matter, carbonate, gravel, and silt. However, A. gibbosa thrives in more silt, while A. angulatus prefers medium to very fine sand. Textularia earlandi correlates well with gravel, and Pseudononion atlanticum and Quinqueloculina patagonica have a positive correlation with high organic matter levels. Buccella peruviana, Quinqueloculina lamarckiana, and Textularia earlandi dominate where organic matter, carbonate, gravel, and silt levels are higher.
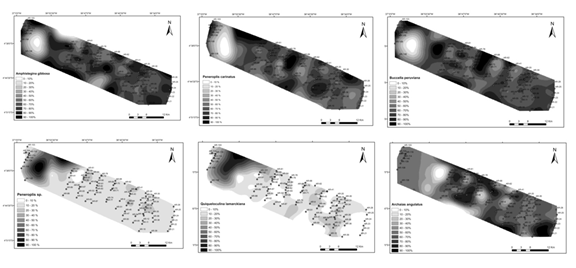
Figure 10 Patterns of foraminiferal species (Amphistegina gibbosa, Peneroplis carinatus, Buccella peruviana, Peneroplis sp., Quinqueloculina lamarckiana, Archaias angulatus) showing a similar distribution in the Açu reef area.8

Figure 11 Distribution of competing foraminiferal species in the Açu reef area. Retired from Eichler et al.8
Sedimentological data from Açu
Analysis of sedimentological material collected from the marine substrate of the outer shelf portion revealed variations in the sizes of unconsolidated grains, ranging from gravel to very fine sand and it is illustrated in Table 7, Figure 12. The granulometry corresponding to the silt and mud fractions were not very representative, while, in greater proportions, the gravel, coarse sand and medium sand fractions were recognized. Through the granulometry and nature of its material – biochemical, siliciclastic or mixed – it is possible to recognize many sedimentary facies, as well as subdivide them into a group of subfacies based, as main parameters, on the calcium carbonate content and granulometry.
Table 7 Açu incised Valley stations and sediment components. Modified from Eichler et al.8
Organic matter content exceeded its importance in correlating with biotic data, not interfering in the characterization and/or definition of facies/subfacies.
The primary structure supporting the living corals and other reef inhabitants at Pirangi consists of sponges and algae rather than sedimentary rocks formed by coral skeletons, as seen in other global reefs like the Great Barrier Reef.9 Maracajaú's epibenthic assemblages inhabit sandstone beach rock,39,40 while Açu's reef communities rest on fossil reefs built by hermatypic corals, sponges, and green algae.23 Reefs from Brazil differ from those in the Caribbean, Indian, and Pacific Oceans in their faunal composition, harboring fewer and several endemic coral species.41 Though not the primary builders, stony corals are present throughout the northeastern region under stressful conditions of temperature, turbidity, and salinity.42 Brazilian reefs often comprise arenitic rock covered by coral and calcareous algae, with macroalgae like brown and green algae competing for space. This pattern, observed at Pirangi, indicates higher macroalgal abundance and low coral cover.29,43–46 However, macro algae dominance does not always signify poor health, as seen in Hawaiian reefs where they contribute positively to reef health.47,48
Sponges provide essential substrate cover, creating complex habitats that enhance local biodiversity.39,49 They host symbionts like young lobsters and small crustaceans, offering protection and food sources.50–52
Symbiont-bearing benthic foraminifera also offer valuable indicators of reef health.53–55 This study focuses on the abundance variations of Amphistegina gibbosa and Amphisorus hemprichii, with Amphistegina gibbosa being more abundant overall. In tourist-impacted areas, A. gibbosa shows significant decline, while Amphisorus hemprichii thrives in seagrass and on soft corals, consistent with previous findings.56–58 Foraminiferal shells from Pirangi often show signs of environmental stress and reworking, similar to findings in the Abrolhos reefs, indicating possible riverine influences.59,60 In contrast, Maracajaú's reefs, farther from the coast, display better-preserved foraminiferal communities. Data from Açu indicate high-quality water, with well-preserved SBF suggesting minimal anthropogenic impact.11 The diverse and abundant foraminiferal assemblages in Açu reflect high organic matter and CaCO3 levels, correlating with healthy carbonate sedimentary facies.7 These findings underline the importance of foraminifera as bio indicators and the need for continued environmental monitoring and protection of reef ecosystems.61–64
The prevalence of smaller, stress-tolerant foraminiferal species and a low presence of symbiont-bearing taxa in at least two of the three reef areas indicate suboptimal conditions for the SBF. Pirangi reefs, in particular, exhibit a notably low species count compared to other Brazilian reefs, such as Abrolhos. Maracajaú's patch reef system appears healthier but is negatively impacted by tourist activities causing physical damage. Amphistegina gibbosa is absent in heavily trafficked areas, though Amphisorus hemprichii is sometimes present. Some foraminiferal assemblages from Rio Grande do Norte seafloor sediments are clearly relict.65
Hard substrates and coarse sediment, rather than water depth, influence the distribution of symbiont-bearing foraminiferal species. Organic matter and CaCO3 content are the main variables affecting species diversity, with mud having minimal impact. CaCO3 levels decrease with increased siliciclastic material, leading to greater species dominance but reduced individual diversity. Conversely, higher carbonate content areas exhibit increased diversity. Carbonate or mixed sedimentation environments have high carbonate content, with Amphistegina gibbosa and Peneroplis carinatus as indicators. In muddy, siliciclastic sedimentation zones, Quinqueloculina lamarckiana dominates with low diversity. Archaias angulatus and Amphisorus hemprichii increase in mixed sediment areas as Amphistegina and Peneroplis decrease.66
Opportunistic and dominant species concentrate in low CaCO3 environments, while symbiotic associations prevail in higher carbonate areas. Greater diversity of symbiotic species indicates better environmental quality favoring carbonate production. The presence of Buccella peruviana, Globigerinoides rubra, Quinqueloculina patagonica, Peneroplis pertusus, and Amphisorus hemprichii in Açu suggests less cold water intrusion and nutrient-enriched upwelling zones. Globigerioides rubra, indicative of deeper, cooler marine environments, may also signal upwelling west of the study area. The manuscript's interpretation of Globigerinoides ruber in relation to environmental conditions needs clarification to resolve the apparent contradiction. Globigerinoides ruber is indeed a planktic foraminifer predominantly found in tropical-subtropical surface waters, as highlighted by Schiebel and Hemleben in their comprehensive review of planktic foraminifera. This species thrives in warm, oligotrophic waters, often indicating reduced cold-water intrusion.
peruviana’s central distribution correlates with CaCO3 and organic matter, indicating a nutrient-rich upwelling zone. Robust-shelled species like Amphistegina and Quinqueloculina confirm the high-energy, high-current outer shelf environment.
Factors such as the presence of symbiont species, species diversity, and direct correlations to carbonate facies, along with organic matter distribution, support the presence of a healthy, carbonate-producing environment linked to shelf-breaking processes and outer shelf reef interactions.
CAPES and, in particular, the project “Processos Oceanográficos Na Quebra Da Plataforma Continental do Nordeste Brasileiro: Fundamentos Científicos para o Planejamento Espacial Marinho” (Edital Ciências do Mar 2 no. 43/2013, Proj. 23038.004320/2014-11) is thanked for a postdoctoral fellowship for Eichler at UFRN-Brazil. We also acknowledge CAPES and CNPq for the Professor Visitante Especial Fellowship awarded (Proj. 151/2012 and AUX PE 242/2013) in the Science without Frontiers Program. We are grateful to the National Institute of Science and Technology of Tropical Marine Environments INCT AmbTropic. We also thank PROBRAL 337/10 (CAPES/DAAD) for the short postdoctoral fellowship of Eichler (grant n. 8116-12-1) at Kiel University, and to CNPq (Researcher Grant PQ number 303481/09-9) and MCTI/CNPq no. 23/2011 - Apoio Técnico para Fortalecimento da Paleontologia Nacional (grant number 552976/2011-3).
The authors declare that there are no conflicts of interest.

©2024 Eichler, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.