Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 1
Caspian Sea Ecology Research Center (CSERC), Iranian Fisheries Science Research Institute (IFSRI), Agriculture Research, Education and Extension Organization (AREEO), Iran
Correspondence: Seyyed Mohammad Vahid Farabi, Caspian Sea Ecology Research Center (CSERC), Iranian Fisheries Science Research Institute (IFSRI), Agriculture Research, Education and Extension Organization (AREEO), Mazandaran province, Sari, Iran
Received: March 19, 2024 | Published: April 12, 2024
Citation: Farabi SMV, Safari R, Ghiasi M, et al. Physiological deceiving of Rutilus kutum fry with a saline diet in freshwater to stimulate the ion-osmotic regulation system and direct transfer to the Caspian Sea. J Aquac Mar Biol. 2024;13(1):23-29. DOI: 10.15406/jamb.2024.13.00392
Caspian Rutilus kutum with the scientific name of Rutilus Kutum is one of the most important economic fish in the southern region of the Caspian Sea. Millions of juvenile fish are released into the rivers each year to resource reconstruct sea fish. At this time the rivers are not suitable due to water pollution for the release of juveniles. One of the most common methods in the world is the readiness of juveniles to transfer directly from freshwater to seawater by stimulating its osmotic regulation system in freshwater. This study was carried out on the feasibility of increasing the physiological capacity of juvenile fish with a salt diet in freshwater. Fish fry were fed and reared for 35 days in two earthen ponds with commercial feed up to a weight of 1±0.1 grams. In an earthen pond and the last 15 days of the rearing period, the fry was fed with a 2% salt diet. Then the fish in 2 experimental groups and 3 repetitions were directly introduced to the Caspian Sea water. Fish gill and kidney tissues were sampled before and after the experiment under salt stress. The results of the biometric examination of the fry showed that there was no disturbance in their growth process by feeding them with a salty diet. The relationship between the length and weight of fry fed with a salty diet (F1) and without feeding with a salty diet (F2) had a high correlation coefficient (0.99 and 0.98, respectively). The condition factor was no significant difference between groups F1 and F2 (P>0.05). As well as the survival rate of juvenile fish was 100% during the assay period in brackish water (15 days) and there was no adverse change in the tissues in the two groups. Also, investigation of gill tissue sections showed that after introduction to brackish water, the chloride cells were observed in all two groups. However, fish fed with a salty diet in fresh water and after exposure to brackish water had a larger size and more chloride cells than the control group. Therefore, there is the possibility of directly releasing of juveniles about one gram into the Caspian Sea based on their physiological ability.
Keywords: Rutilus Kutum, Caspian Sea, salty diet, gill, kidney
Fishing has declined significantly over the past three decades in the southern Caspian Sea. However, the stocks of Rutilus Kutum (Kamensky, 1901) were partially preserved due to artificial reproduction in the fish stocks recovery program of the Fisheries Organization of Iran. For this reason, Rutilus Kutum is currently the most important species of fish that can be caught, and in terms of protein supply, employment, and income generation of people in the southern Caspian Sea region is very important compared to other native Caspian fish.1 Rutilus Kutum is a member of the family Cyprinidae from anadromous fish. This fish for reproduction are migrating to freshwater river in the spring.2 Rutilus Kutum lives in temperate regions with longitudes of 32 to 67 E in the Northern Hemisphere and live in the Caspian Sea basin, the rivers of the northern Black Sea and Azov Sea.3
All over the world, programs have been developed for the mass production of wild fish in breeding centers and the release of young fish into natural environments to increase the amount of commercial catch.4 Rutilus kutum needs protection in current conditions due to changes in the environment and spawning places and river pollution.5 In 1982-83, the population of Rutilus kutum was greatly reduced and its catch in Iranian waters reached 1000 tons. However, with the continuation of the process of artificial reproduction, its population has been relatively restored.2 Currently, Rutilus kutum is classified in the IUCN Red List with the symbol LC (least concern). Therefore, due to the reduction of Rutilus kutum stocks, the reproduction and cultivation of this valuable species in order to restore its stocks is of particular importance in many countries around the Caspian Sea, and it is considered as the main solution in increasing Rutilus kutum stocks.1,6
According to the statistics published by the Iranian Fisheries Organization,7 the number of Rutilus kutum fry released in the southern region of the Caspian Sea is as shown in Figure 1.7

Figure 1 Withe fish or Caspian kutum (Rutilus Kutum, Kamensky, 1901).1
1Photo of Rutilus kutum taken from the book " Mahi Sefid (Rutilus kutum), Jewel of the Caspian Sea" (Khanipour and Valipour, 2009)
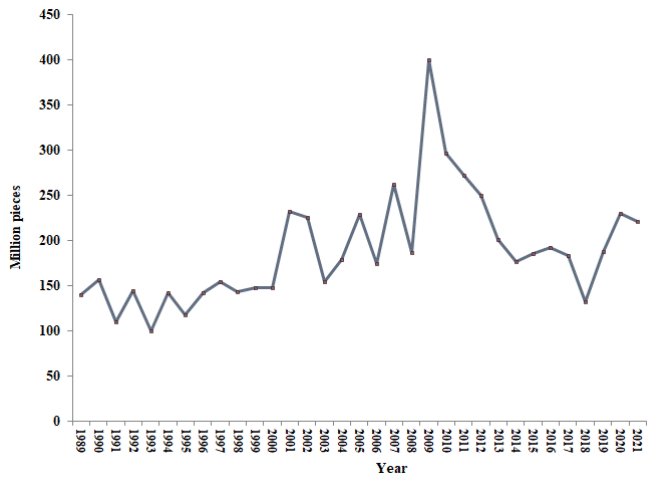
Figure 1 The number of Rutilus kutum fry release during the years 1368-1399 in the southern region of the Caspian Sea.
The most important effective factors in the adaptation of fry to environmental conditions in the process of restoring natural resources include: size, quality of physiology, density of rearing, transportation of fry, techniques of their release into natural environments.8–10
Salinity is considered one of the important factors in the environment of fishes, and in migration, fishes need a mechanism for adaptation or ionic-osmotic regulation in new conditions along with changes in the salinity of the environment.11 Therefore, by stimulating and activating the ion-osmotic regulation system in Rutilus kutum before entering the brackish water of the sea, their widespread mortality can be prevented. Because the criterion of preparation for the migration of fish from fresh water to the sea is physiological changes, especially the preference for salt water and ionic-osmotic regulation.
Factors such as the size and age of the fish, the environmental conditions and the ability of each fish are effective in the adaptation of fish to salt water, and it scaues some changes in the gill and kidney tissue, hormonal, ionic, enzymatic, metabolic, and blood factors in the fish. These changes provide the physiological preparation of the fish for ion-osmotic regulation.12–16 Different sources have reported the effect of water salinity on the osmotic regulation system of fish from 24, 48, 72, 96, 168 hours to 14 days.17–19
In this study, a different method was used to adapt Rutilus kutum to brackish water. Feeding fish with salty diet is one of the most common methods in the world to stimulate the ion-osmotic regulation system and the physiological readiness of Chinook salmon (Oncorhynchus tshawytsha) fry in direct transfer from fresh water to salt water.20
The use of salt diet in fresh water environment for initial preparation and adaptation of fish to salt water by Salman and Eddy,21 Smith et al.,22 Trombetti et al.,23 Perry et al.,24 on rainbow trout (Oncorhynchus mykiss) fry, Pellertier and Besner25 on Brook charr (Salvelinus fontinalis) fry, Fontainhas-Fernandes et al.,26 Lim et al.27 on Oreochromis niloticus fry, Applebaum and Jesuarockiaraj28 on Gilthead sea bream (Sparus aurata L.) fry, Keshavanath et al.29 on Tambaqui (Colossoma macropomum) fry, Santos et al.30 on cobia fry, Mzengereza and Kang’ombe31 on Oreochromis shiranus fry. The results of their investigation showed that the salt diet has deceived and stimulated the organs that are effective in ion-osmotic regulation.
The purpose of this research is the physiological preparation of Rutilus kutum fry from artificial reproduction by feeding them with salt diet and the possibility of stimulating the ionic-osmotic regulation system of fish in fresh water (fish lure) in order to directly release them into the brackish water of the Caspian Sea.
Study area
The southern region of the Caspian Sea as the habitat of the Rutilus kutum of the Caspian Sea with a moderate climate was used as the place for the survey. Juveniles fish were obtained from Shahid Marjani fish reproduction and restoration center in Mazandaran province (Iran). Larvae were fed (salt diet) and reared up to the juvenile fish stage of 1±0.1 g in fresh water. The salinity stress test and the bioassay test were carried out in the water of the southern shore of the Caspian Sea and inside three floating cages.
Fish fry were fed and reared in 2 earthen ponds with the same density of one million pieces per hectare for 35 days with the commercial feed of Mazandaran livestock and aquatic Company (Table 1) at the rate of 20% of body weight. However, the fish were fed with salt diet (feed containing 2% sodium chloride salt) in an earthen pond in the last 15 days of the rearing period.29–33
|
Approximate analysis |
Amounts (%) |
|
Moisture |
10±2 |
|
Ash |
35±2 |
|
Crude protein |
10±2 |
|
Crude fat |
15±2 |
|
Fiber |
4±1 |
|
Free nitrogen |
20 |
|
Digestible energy |
3300 kcal/kg |
Table 1 Analysis of commercial powder feed of Rutilus kutum fish (Mazandaran livestock and aquatic Company)
*Two present (2%) of salt was added to the formula feed to prepare salty diet.
After catching the fry from the two earthen ponds (F1: fish fed with salt diet and F2: without salt diet), they were transported to the test site on the southern coast of the Caspian Sea in the port of Amirabad (53.37415 East, 36.85607 North) with special transport tankers were transferred. Then counting and treatment was done in 2 groups and they were directly introduced to the brackish water of the Caspian Sea in floating cages (Figure 2), (Table 2).
|
Environment |
Weight (gram) |
Experimental treatment |
Density (N/m3) |
|
Caspian Sea Brackish water |
1±0.1 |
Fish fed with a 2% salt diet in fresh water |
300 |
|
Fish fed without salt supplement |
Table 2 Transfer of Rutilus kutum fry to experimental treatments under salinity stress to floating cages in Caspian Sea water
The fry were kept in brackish water of the Caspian Sea for 15 days and fed with a diet without salt supplementation at a rate of 20% of body weight.17–19
Biometry of fry
The relationship between length (fork) and weight (total) of juvenile fishes in different repetitions was determined according to Formula 1.34
1:
Then the t test was used to confirm the b value obtained from the logarithmic Formula 2 for comparison with the isometric value (b=3).35
2:
W: total weight (grams), L: fork length (cm), a and b: regression coefficients between length and weight), s.d.(x): standard deviation of logarithm of fork length, s.d.(y): standard deviation of logarithm of total weight , n: sample number, r: correlation coefficient
The Condition Factor (CF) of fish was calculated by Fulton's equation according to Formula 3.36
3:
Wf: final weight, L: fork length
The specific growth factor was calculated from the logarithm of the weight of the fish compared to the rearing days according to Formula 4.37
4:
t: number of rearing days, Wi: initial weight (grams), Wf: final weight (grams)
The percentage of survival was calculated from the ratio of the number of live fish remaining in the earthen pond or experimental units to the number of initially stocked fish, multiplied by 100 according to Formula 5.38
5:
Ni: number of stocked fish, Nf: final number of live fish
Laboratory study
In order to measure the stimulation of ion-osmotic regulation system, the histology of two kidney organs and gills of fish were examined at the end of the storage period in fresh water (earthen pools) and also in brackish water (Caspian Sea). After preparation, tissue samples were stained with hematoxylin and eosin method.39
Applied histology guide40 and Fish Histopathology Atlas41 were used to detect changes in gill and kidney cells of juvenile fish.
Excel, 2010 software was used to draw the graph and determine the mean and standard error. For the statistical analysis of the data, the statistical program Spss version 23 was used. Kolmogorov-Smirnov test was used to check the normality of data distribution (NIST / SEMATECH, 2010). Also, exponential regression, Pauli test and t-test at 5% level were used for the relationship between length and weight.
Weight and length growth of Rutilus kutum fry in earthen ponds
The weight and length growth of Rutilus kutum in two earthen ponds fed with salt supplement and without salt supplement in 6 stages from March to July was as described in Figure 3.
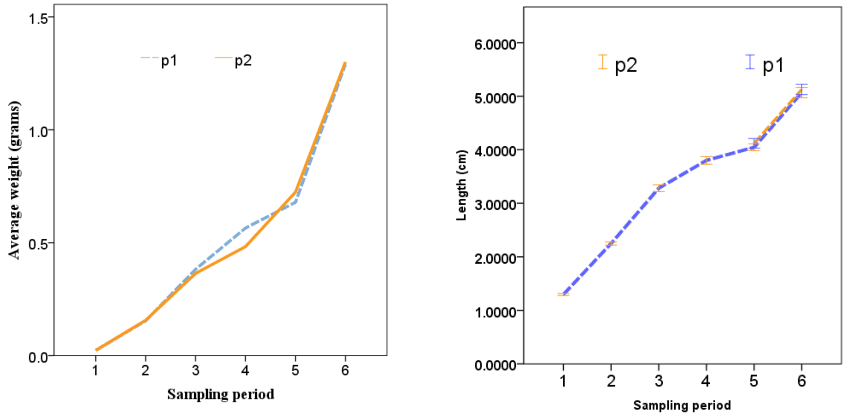
Figure 3 Weight (grams) and length (cm) growth of Rutilus kutum fry in fresh water of earthen ponds (F1: fed with salt supplement, F2: without salt supplement).
The relationship between the length and weight of fry fed with salty diet (F1) and without feeding with salty diet (F2) had a high correlation coefficient (0.99 and 0.98, respectively). Also, the coefficients of length and weight (a and b) of fry were calculated as a: 0.013, b: 2.80 and a: 0.0129, b: 2.78, respectively (Figure 4).
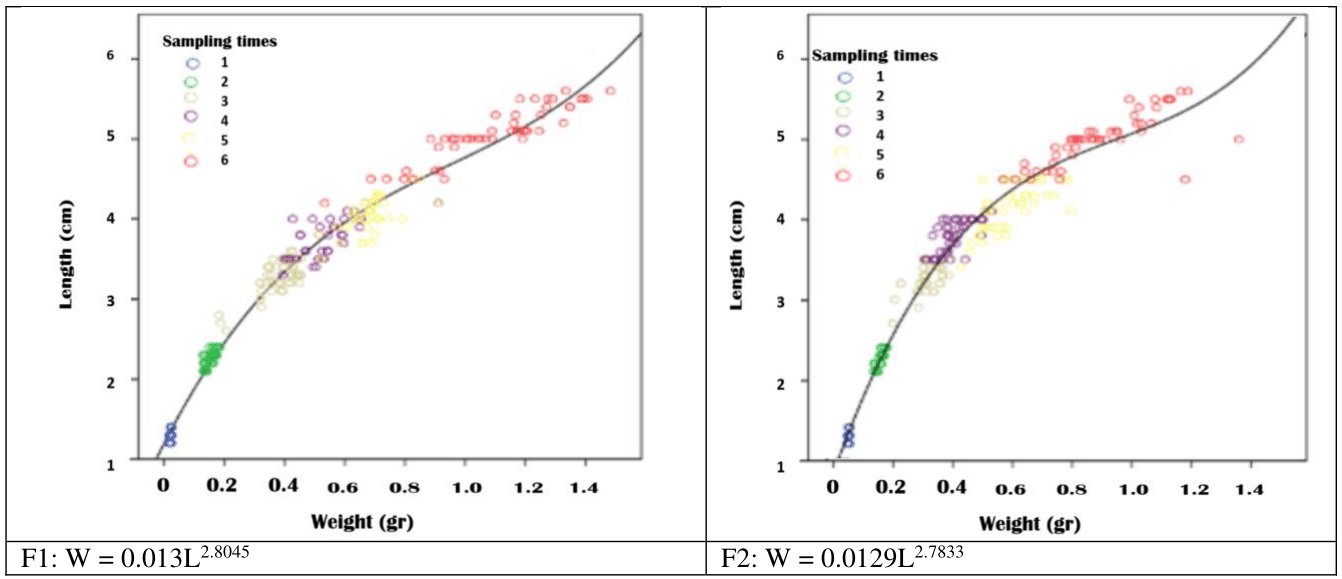
Figure 4 Relationship between the length and weight of Rutilus kutum fry fed with salt diet (F1) and without salt diet (F2) during the rearing period in fresh water of earthen pond.
In the Pauli test and in the t-test with 95% confidence limits, the value of b calculated in the fry of two earthen ponds had a significant difference with the number 3 and it was equal to t=9.45 and t=10.26 respectively, P<0.05. The condition factor in the period of rearing larvae to fry about one gram showed that it has a downward trend with increasing weight (Figure 5) and there was no significant difference between groups F1 and F2 (P>0.05).
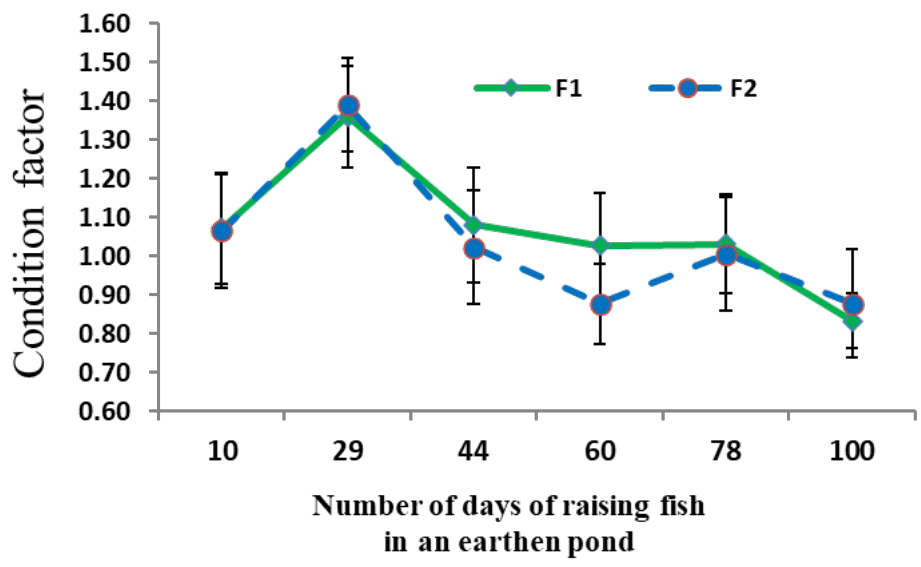
Figure 5 Average (± standard deviation) condition factor of Rutilus kutum fry fed with salt diet (F1) and without salt diet (F2) during the rearing period in fresh water of earthen pond.
The percentage of specific growth rate in the larval to fry rearing period in earthen ponds showed that it had higher values at the beginning and its value decreased at the end of the period (Figure 6).
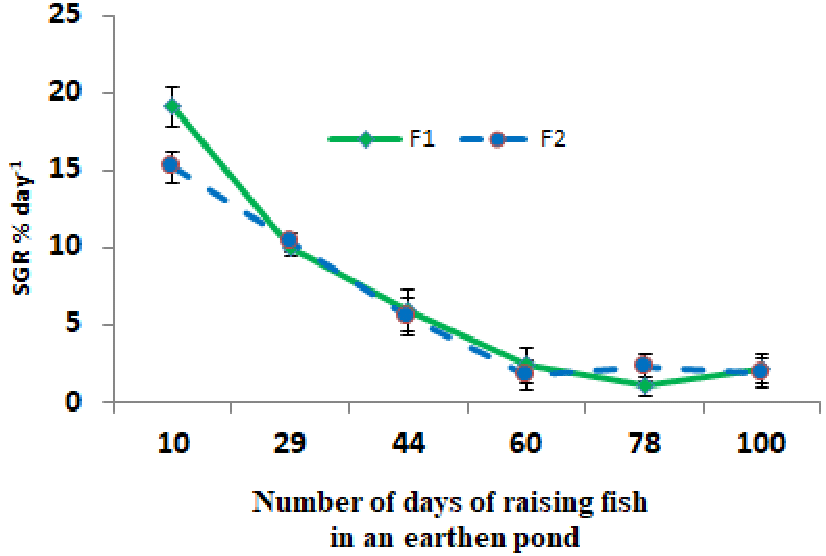
Figure 6 Average (± standard deviation) percentage of specific growth rate in the period of rearing larvae to fry of Rutilus kutum fed with salt diet (F1) and without salt diet (F2) in fresh water of earthen ponds.
Survival test in Caspian Sea water
During the test period and direct introduction of the fry to the Caspian Sea water, no fish death was observed in any of the experimental groups.
Tissue sections of kidney and gill of fish fry in freshwater
In the kidney tissue of fish fry in both groups fed with salt supplement (F1) and without salt supplement (F2) in fresh and brackish water, most of the kidney space is composed of mesonephric tubules, and glomerulus was also observed. The structure of renal tubules (distal and proximal tubules) was completely normal. But there was a slight difference in the size of the glomerulus between F1 and the control group, so that in F1: the glomerulus was slightly wrinkled and there was a distance between the cover of Bowman's capsule and the glomerulus (Figure 7).
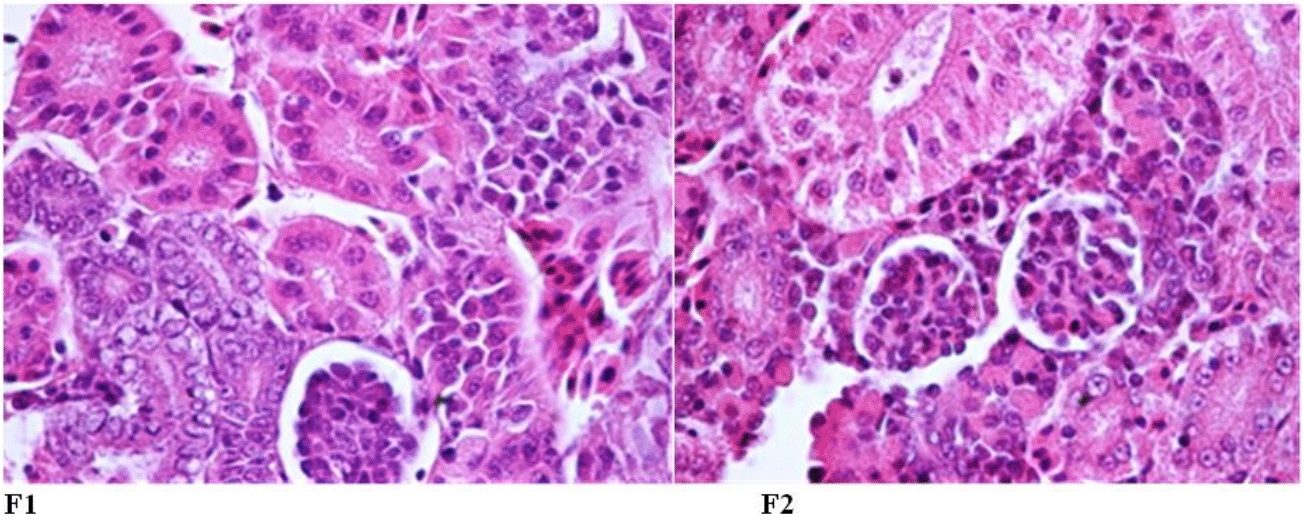
Figure 7 Kidney tissue of Rutilus kutum fry fed with salt diet (F1) and without salt supplement (F2) in fresh water (fixed in 10% formalin and stained with hematoxylin and eosin: 1000×).
At the end of the fish rearing period in fresh water, an increase in the size of cells (hypertrophy) and accumulation of red blood cells (hyperemia) were observed at the base of the gill rakers of fry chloride fed with salt diet (F1) compared to the control (F2), (Figure 8).
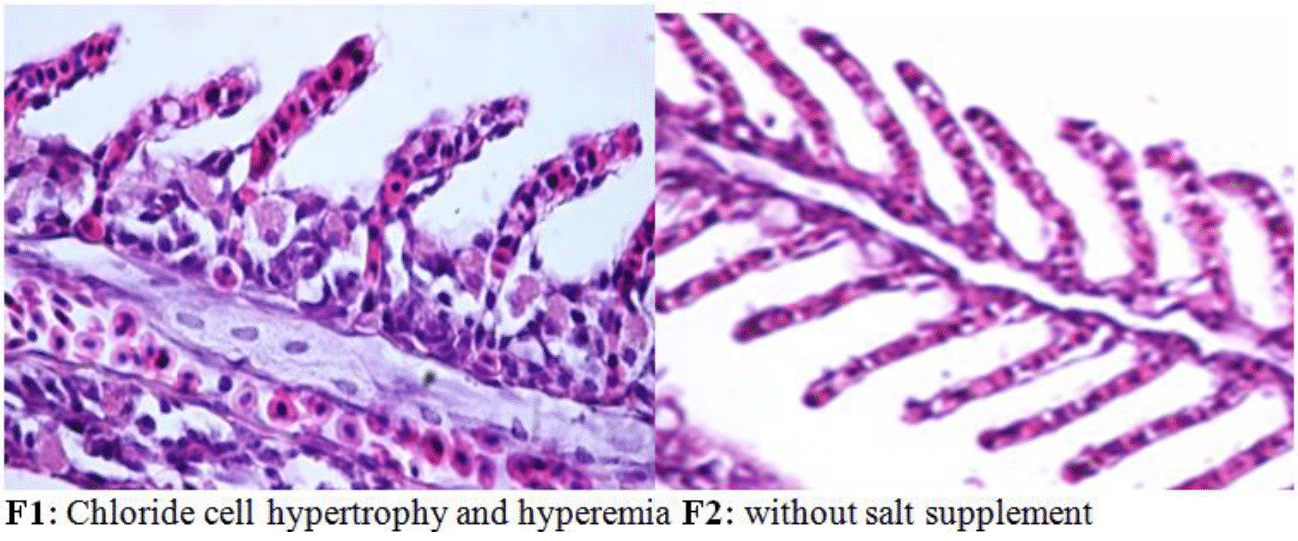
Figure 8 Chloride cells of the gills at the base of the secondary blades of Rutilus kutum fry fed with salt diet (F1) and without salt supplement (F2) in fresh water (fixed in 10% formalin and stained with hematoxylin and eosin: 1000×).
Tissue sections of kidney and gill of fish fry in brackish water
The histological evaluation of the kidneys of Rutilus kutum fry after 10 days of exposure to the brackish water of the Caspian Sea showed that the structure of the kidney was completely normal in both experimental groups fed with salt diet and without salt supplement (Figure 9).
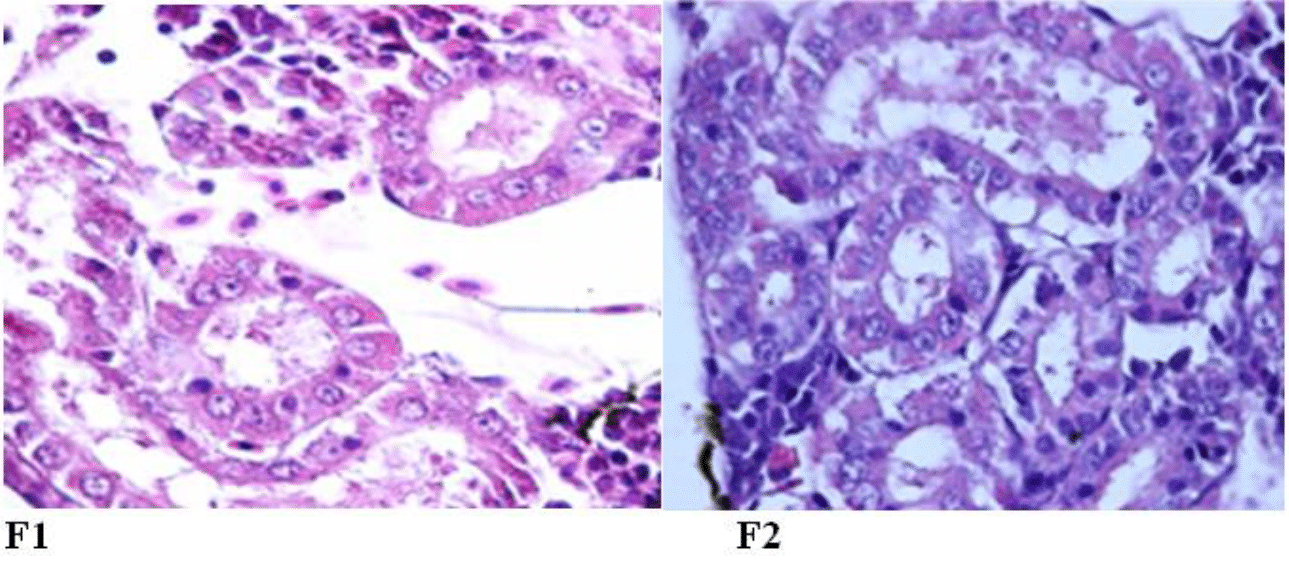
Figure 9 The normal structure of renal tubules of kidney tissue of Rutilus kutum fry fed with salt diet (F1) and without salt supplement (F2) in the brackish water of the Caspian Sea (fixed in 10% formalin and stained with hematoxylin and eosin: 1000×).
An increase in the volume and number (hypertrophy and hyperplasia) of chloride cells as well as the accumulation of red blood cells was observed in the gill rakers of Rutilus kutum fry of the control group and fed with a salt diet after 10 days of exposure to the brackish water of the Caspian Sea. However, the number of chloride cells in the control group was lower (Figure 10).
Rutilus kutum is one of the most important economic bony fishes in the process of stock restoration in the southern region of the Caspian Sea. In the last 30 years, an average of 187.92 million fry7 with a weight of one gram and a length of 5 cm have been released into the rivers of the southern Caspian Sea (Figure 1).1
Physiologically, the preparation of fish fry during migration and transfer from fresh water to salt water and from river to sea is of special importance to resist and adapt to unfavorable environmental conditions and increase their survival. As a result, the weak physiological conditions of fish fry resulting from artificial reproduction to introduce them to a new environment is one of the most important factors in their mortality in the early stages of life. The most of the deaths are related to the first few days or the first few weeks, after release to the new natural environment.42,43 The appropriate age and size of fish fry are considered to be the most important factors in their physiological preparation.9,10 Because when the fish enters from fresh water to salt water, there are changes in the ion-osmotic regulation system of the fish. This process changes the state from hyperosmotic (in fresh water) to hypo-osmotic (in salt water) in fish.44
The ion-osmotic regulation system in fish is an energy-demanding process and is associated with stress, and it causes the activation of the hypothalamus-pituitary axis and affects various organs, especially the kidneys and gills in fish. This process in transferring fish to a new environment (fresh and salt water) causes adaptation and ionic-osmotic balance in fish. If the fish cannot reach the balance with the environmental changes, its survival in the new environment will be disturbed and it will die.13
In this research, fish fry was raised in fresh water for a certain period of time to a suitable weight (one gram) and stimulation of the osmotic-ion regulation system in the gills was done with salt supplement. The relevant organs are activated and the chloride cell size of the gill increased (Figure 8). This process was tested by different scientists with different percentages of salt supplement on different species of fish of different sizes and had similar results.20–30
In this research, the results of the histological examination of the gills of Rutilus kutum showed that the size of chloride cells and their number increased in the group fed with saline diet in fresh water (F1) compared to the control group (F2), (Figure 8). (In the second stage of exposure to salt stress with a weight of one gram in a floating cage in the Caspian Sea, the volume and number of chloride cells increased in both groups (F1 and F2), with the difference that this change was more significant in the F1 group (Figure 10). Because, fish gills have two types of mitochondria-rich cells (MRC) of alpha (α) and beta (β) types, and in adapting to sea salt water, β type cells are lost and α cells are multiplied and transformed, and also Lateral cells become chloride cells.45
The research of Daborn et al.46 showed that during the transfer of fish from fresh water to salt water, gill chloride cells are created from the differentiation of squamous cells, and after contact with salt water, the rate of this differentiation increased and with the passage of time, the number of gill chloride cells increase. Also, Foskett et al.47 showed that after a few days of exposure to salt water, the number of chloride cells reached a constant level and the rate of increase in cell size accelerated. In this study, hyperplasia and hypertrophy of chloride cells were observed in brackish water in experimental treatments.
In general, gill chloride cells undergo changes in their size, number, shape and distribution in response to environmental salinity changes. Also, there is a significant increase in their number and size.48,49 Also, studies have shown that this change occurs with the use of salt diet in fresh water.24,50
On the other hand, the increase in the number of chloride cells increases the thickness of the gill rackers, which can disrupt the transfer of oxygen and carbon dioxide.30 In order to prevent such a phenomenon, blood flow increases in the gill filaments and justifies hyperemia in this part.
Various studies were conducted on Rutilus kutum fry and it indicates the ability of ion-osmotic regulation of Rutilus kutum fry against salinity stress. Imanpour51 and Amiri et al.52 by studying the salinity stress on Rutilus kutum fry showed that there is an ability to adapt in such conditions. Also, Imanpour51 showed that with increasing weight from 0.5 to 3 grams, the number of chloride cells increased significantly. Also, the research of Farabi et al.32 showed that the destruction of gill tissue was observed in some Rutilus kutum fry less than 0.5 g in direct transfer from fresh water to brackish water of the Caspian Sea.
In the Rutilus kutum fry fed with salt diet, protrusions appeared in the cells ready to transform into chloride cells, which was not observed in the control treatment in fresh water environment. However, after introducing the fry to the brackish water, the appearance of chloride cells was observed in both experimental groups. This result is consistent with the research of Mohiseni et al.53 on Rutilus kutum. They showed that Rutilus kutum exposed to brackish water show an increase in the number of gill chloride cells until the fourth day, and then it reaches a relatively constant level until the seventh day. Also, this information is consistent with the studies of Foskett et al.47 in cichlid fish during adaptation to salt water.
Therefore, salt diet on fish fry in fresh water is effective in increasing the volume and number of chloride cells in the gill tissue when exposed to brackish water and causes changes in gill function for better ion-osmotic regulation. Fish kidney is one of the most important organs of fish to adapt to different environments with different salinities.54 In this research, the histological evaluation of kidney tissue in two groups fed with salt diet and without feeding with salt supplement, the condition of proximal and distal renal tubules was completely normal (Figure 7 and 9). The only difference that was observed between F1 and F2 groups was in the diameter size of the glomerulus. In other words, the increase in the space between Bowman's capsule and glomerulus was more in F1 group in fresh water and salt water. A review of studies shows that the glomerulus size in fish is smaller in saltwater than in freshwater.55,56
The structure of the kidney in fresh water did not change in the experimental groups, because the function of the kidney is to excrete divalent ions (Ca+2 and Mg+2). In ionic-osmotic regulation in fresh water environment, monovalent ions (Na+, Cl-) are mainly absorbed in the gills to be transferred to the blood serum of fishes, and more water is excreted from the kidneys. On the other hand, in the saltwater environment, monovalent ions are mainly secreted from the gills and divalent ions from the kidneys.48
In both experimental groups, an increase in the inner cavity of the renal tubules (proximal and distal) and a decrease in the diameter of the glomeruli compared to fresh water were observed in both experimental groups in brackish water (Figure 9) and this change was done due to adaptation to the new environment.
In the research done by Stoskopf,57 it showed that the size of glomeruli in fishes is different, but bony freshwater fishes have more and bigger glomeruli than marine fishes. In this research, by changing the environment of baby fish from fresh water to brackish water of the Caspian Sea, the structure of their kidneys changed. But in the use of salt supplement in fresh water, no change was observed in the kidney tissue.58–61
In this research, the process of physiological changes was accelerated by deceiving the fry by feeding them with a salty diet, and the fry had a better physiological ability to exposure salt stress (Bio assay test). Based on this, it is possible to directly release one-gram fry fed with salt ration into the Caspian Sea instead of entering polluted and sometimes low-water rivers at the time of release. For further research, it is suggested that clinical tests (serological and biochemical blood) be conducted to ensure the effect of salt on fish physiology.
I am very grateful to the director, assistants and respected colleagues at the Caspian Sea Ecology Research center. Also, I am very grateful to the honorable director of the Mazandaran Fisheries administration for providing financial support for the research.
The authors declare that there are no conflicts of interest.

©2024 Farabi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.