Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 6
1Department of Fishery, Faculty of Marine Science and Fishery, Indonesia
2Department of Environment and Agriculture, Sustainable Aquatic Resources and Biotechnology, Australia
3Department of Agriculture and Food Western Australia, Animal Health Laboratories, Australia
Correspondence: Irfan Ambas, Department of Fishery, Faculty of Marine Science and Fishery, Hasanuddin University, Indonesia
Received: December 04, 2019 | Published: December 20, 2019
Citation: Ambas I, Fotedar R, Buller N. Performance of marron (Cheraxcainii) origin probiotic Bacillus mycoides in earthen commercial marron ponds. J Aquac Mar Biol. 2019;8(6):246-252. DOI: 10.15406/jamb.2019.08.00268
The present study evaluated the performance of marron (Cheraxcainii) origin probiotic Bacillus mycoides post laboratory scale trials by measuring total haemocyte counts (THC),hepatosomatic indices (HSi), intestinal bacteria population, gluthathionine peroxide (GPx) enzyme activity, and survival rate (SR)of marron.
The probiotic was supplemented to a commercial marron feed (used as basal diet) at 108CFU/mL and given to marron during eleven months feeding trial using a commercial marron ponds (10x15m2). The ponds were stock with marron juveniles at 3000/pond. The probiotic feed was prepared weekly to ensure its freshness and viability, then kept in refrigerator before using.
The results suggested the THC, His andthe intestinal bacteria population of marronfed probiotic supplemented diet were significantly higher (P<0.05) compared to THC, HSi and intestinal bacteria population of basal diet fed marron both on day 90th and day 160th.The GPx enzyme activity was detected also significantly higher (P<0.05) in marron fed with the probiotic diet both on day 90th and termination of the feeding trial.At termination of the trial, survival ofmarron fed a diet supplemented probiotic was significantly higher (74.80±2.52%) than that of marron fed a basal diet(66.15±6.33%).
In brief, the customized marron origin probiotic B. mycoides worked effectively in vivo (commercial marron ponds) as indicated by a significant improvement of marron immunity and health status (THC, GPx enzyme activity, intestinal bacteria population and HSi) which in turn enhanced survival rates when compared to basal diet fed marron.
Keywords marron, probiotic, THC, HSi, SR
HSi, hepatosomatic indices; THC, total haemocyte counts; GPx, gluthathionine peroxide
Today, it has been widely accepted that probiotics play a significant role in aquaculture1–4 as an ecofriendly methodfor disease control for sustainable aquaculture,5 however there has been only a few in vivo studies on the use of probiotics in a controlled environment.6 In screening a probiotic candidate,an in vivo test is essential7–10 as in vivo physiology is more complex and different from in vitro monoculture6 No study has ever compared probiotic beneficial effectsin vitro and in vivo.11
The complexities encountered by an added probiotic under outdoor conditions include (i) the uncertainty for the probiotic to remain viable in an aquatic environment3(ii) interaction with other strains in the host environment12,13 (iii) selection process by the host gastrointestinal tract(GIT)14 (iv) competition with the indigenous GIT inhabitants10 (v) viability during storage.15 To complicate the issue, not all of the authors examined the viability of the probiotics during the feed preparations after the microbial cells have been added to the fee.3 For these reasons, the marron origin (host GIT and its environment) with favourable probiotic properties is preferable by most of the authors for an ideal candidate1,2,7,16-18as its efficacy is likely to be highest in the host and particularly in its natural environment.7,19
Bacillusmycoidesis a predominated bacterium isolated from a number of healthy adult farmed marron GIT that exhibit favourable probiotic properties such as non-pathogenicity to marron, antagonism ability towards common crayfish pathogens (Vibrio mimicus and V. cholerae non-01), exhibition of a diverse enzyme profiles and non-susceptible to the majority of antibiotics tested20 improved immunity21 and the gastrointestinal health status of marron.22
To date, evaluating probiotic performance in vivo studies is limited to tiger shrimp Penaeusmonodon,23 shrimp Litopenaeusvannamei24 and beluga Husohuso.25 When evaluating probioticsin vivo for its nutritional benefit outcomes in aquatic animals, the probiotic candidates should be added to the diet and its effect evaluated on the growth and/or physiological status of the animals.7 The present study examined in vivo performance of customized probiotic B mycoides in commercial marron ponds by counting the total haemocyte (THC), hepatosomatic indices (HSi), intestinal bacteria population, gluthathionine peroxide (GPx) enzyme activity, survival rate and marron pond productivity.
The present study was conducted at an existing commercial marron farm located at 432 Boorara Road, Nortchcliffe Western Australia 6262 (Latitude -34.66001 N; Longitude 116°9' 49.644W). Six of the 900m2 existing commercial marron ponds with an average depth of between 1.6m to 1.7m were used for the dietary supplemented probioticfeeding trial. As the marron production operation in these commercial ponds was already underway before the commencement of the trial, the commencement of this feeding trial was reflected by shifting the existing marron diets to the test diets (probiotic B. mycoides supplemented diet and the basal diet).
The commercial marron diet supplied by specialty feeds, Glen Forrest Western Australia was used as a basal diet, which was used during previous laboratory scale studies. The proximate composition of the basal diet was: 26% crude protein, 9% crude fat and 5% crude ash.
Supplementation of the probiotic to the basal diet followed the established method.26A pure colony of the isolate was grown on blood agar (BA) plates and incubated overnight at 25oC.The overnight growth inoculum was diluted into 20mL of sterilized normal saline before being sprayed onto the basal diet at a concentration of 108CFU/g of feed and then immediately covered with aluminium foil and stored in a refrigerator at 4oC to avoid bacterial growth. The probiotic was supplemented at 108CFU/g of feed and performed on a weekly basis to maintain freshness.
The concentration (CFU/mL) of the probiotic bacterium sprayed onto the feed was determined using an established method27 where optical density (Spectrophotometer, BOECO S-20, Hamburg, Germany) correlates to the bacterial concentration (CFU/mL) and confirmed by performing a total bacterial count using BA plates.28
Feeding was performed once per day in the late afternoon and adjusted weekly after weight sub-sampling of the marron from each pond, which referred to demand feeding rates obtained from The Second Pemberton Grow Out data set 1990-1993, Department of Fisheries Western Australia.
Most of the parameters measured in the present study useda comparable size of the two treatment groups such as hepatosomatic indices (HSi), intestinal bacteria population, total haemocyte counts (THC), gluthathionine peroxide (GPx) enzyme activity, except for the survival rates andmarron productivity in marron pond.
Data collection was performed at day 90th, day 160th and at the harvest (day 310th) for determination of the survival rate, pond production and GPx enzyme activity.
The total haemocyte count was measured following the established methods used for western rock lobsters Panuliruscygnus.29 The haemocyte samples preparation was performed on the farm site and mixed with an anticoagulant at a ratio of 1:1, injected into 2mL cuvette tubes then kept in an iced cool box before taking to the laboratory for THC determination.
In brief, 0.5mL of haemolymph and anticoagulant mixture was inserted into a haemocytometer (The Neubauer Enhanced Line, Munich, Germany) counting chamber and immediately viewed under 100-fold magnification on a camera-equipped microscope and images were taken for THC. Subsequently, the cells were counted in both grids, and the mean was used as the haemocyte count.The total haemocyte count was calculated as THC=(cells counted x dilution factorx1000)/volume of grid (0.1mm3).
The hepatosomatic index (HSi) of marron fed basal and B. mycoides supplemented diets were calculated as per established equations30,31 In brief, the hepatopancreas of marron from each treatment group were removed, placed in foil and weighed. Determination of the hepatosomatic indices (HSi) used the following equation;
HSi= Wwhx 100 Wt-1Where;
HSi= Wet hepatosomatic indices (%)
Wwh=Weight of wet hepatopancreas (g)
Wt=Total weight of marron (g)
The intestinal bacterial population of marron from the different feeding groups was determined following our previous work.22 Before aseptic removal of the GIT, the marron was anaesthetized by placing the animal at -20oC for 5 minutes. Subsequently, the dorsal shell was cut-off from tail to head until the intestines were exposed, then the intestine was collected and placed in a sterilised pestle, weighed and homogenised. The homogenates of intestines were diluted serially (from 10-1 to 10-6) using a sterile normal saline. Fifty microliters of each serial dilution was inoculated onto a blood agar (BA) plate and incubated overnight in a CO2 incubator at 25oC. A colony count was performed for each dilution to determine the total number of aerobic bacteria.28
The gluthathionine peroxide enzyme activity was determined followed the established method.32 In brief, the marron muscle tissue was diluted with a physiological saline at a ratio of 1:1 and stored at 4oC until used.To calculate GPxactivity, 0.2mL muscle tissue homogenates (homogenized in 0.4M sodium phosphate buffer, pH 7.0), 0.1mL 10mM sodium azide, 0.2mL 0.2mM reduced glutathione, and 0.1mL 0.2mM hydrogen peroxide were mixed, then incubated for 10 minutes at 37°C after which 0.4mL of 10% trichloroacetic acid (TCA) was added to stop the reaction.Subsequently, the mixtures were centrifuged at 3200 rpm for 20 minutes. The supernatant was assayed for glutathione content using Ellman’s reagent (9.8mg 5,5′-dithiobis-[2-nitrobenzoic acid] [DTNB] in 100mL 0.1% sodium citrate). The GPx enzymes activity of the samples was measured at the Biochemistry Laboratory, Department of Agriculture and Food, Western Australia. The GPx activity was expressed as micrograms of GSH consumed per minute per milligram of protein.
The survival rate of marron from each pond and treatment group was measured using the established equation as follows;
Where;
SR=survival rate (%)
Nt=No of marron at measurement (ind)
No=No of marron at initial stocking
In addition, the pond production from each pond was determined by counting and weighing total marron at harvest using the following equation;
Pond production (kg/m2)=Total weight (kg)/pond size (m2)
Whereas the average marron weight at harvest was calculated as follow;
Mean weight (g)=Tw/Tn
Where Tw=total weight of marron each pond (g)
Tn=Total number of marron (ind)
The temperature fluctuation was recorded by placing a temperature data logger (Onset HOBO) in each pond.In addition, each pond was equipped with two paddle wheels to ensure sufficient dissolved oxygen especially during critical periods.
The data were analysed using T-test Microsoft Excel for windows version 2010. The difference of means between the two treatment groups was determined at 0.05 levelof significance.
TheTHC of marron fed basal and probiotic, B. mycoides supplemented diets was not significantly different (P>0.05) at day 90th of marron rearing, however on day 160ththe THC ofprobiotic diet fed marron was significantly higher (P<0.05) than the THC of basal diet fed marron (Figure 1).
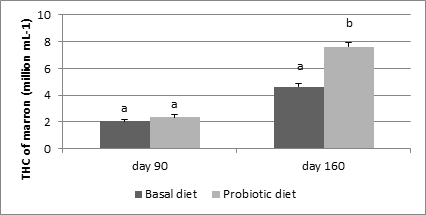
Figure 1 THC of marron (million cells mL-1) fed basal and probiotic diets
*Different letters over bars indicates significantly different at 0.05.
In the present study, the wet hepatosomatic indices (HSi) of probiotic diet fed marron was significantly higher (P<0.05) both at day 90th and day 160th of measurements (Figure 2) than the HSi of basal diet fed marron. The HSi of basal diet fed marron was lower at day 90th than the HSi at day 160th.

Figure 2 Hepatosomatic indices (HSI) of marron fed basal and probiotic diets.
*Different letters over bars indicates significantly different at 0.05.
Supplementation of a marron-origin probiotic, B. mycoidesto thediet significantly (P<0.05) improved the intestinal bacteria population of marron compared to intestinal bacteria population of the basal diet fed marron, both on day 90th and day 160th. In addition, there is an increase of intestinal bacteria population in both treatment groups on day 160thcompared to day 90th (Figure 3).
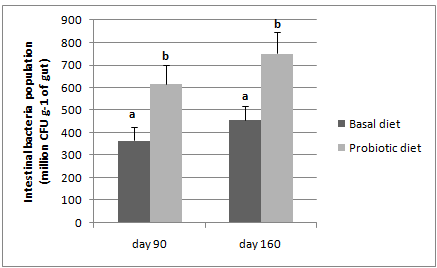
Figure 3 Intestinal bacteria population of basal and probiotic diets fed marron.
*Different letters over bars indicates significantly different at 0.05.
The GPx enzyme activity was significantly higher (P<0.05) in the marron tail muscle tissue fed with the probiotic B. mycoides supplemented diet compared to the GPx acitivity of basal diet fed marron both on day 90th and termination of the feeding trial (Figure 4).
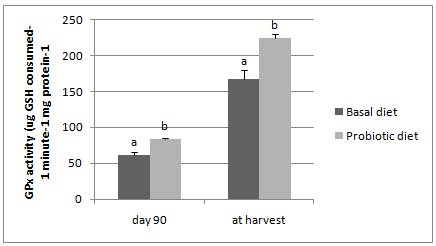
Figure 4 GPx enzyme activity in tissue muscle of basal and probiotic diets fed mar
*Different letters over bars indicates significantly different at 0.05.
The present study demonstrated that the survival rate (%) of probiotic fed marron ranged between 74.80±2.52 (%), which was significantly higher (P<0.05) than the survival rate of marron from the ponds fed with basal diets (66.15±6.33%).
Meanwhile, the average pond production of the basal diet was 258.3±32.6g/m2 whereas the average pond production of the probiotic diet fed marron was 215±26.1g/m2(Table 1).
|
Basal diet |
Probiotic diet |
||||
1 |
2 |
3 |
1 |
2 |
3 |
|
Initial stocking (n) |
3200 |
3000 |
3200 |
3100 |
3200 |
3200 |
Total harvest (n) |
1662 |
2287 |
2249 |
2139 |
2472 |
2474 |
Survival (%) |
51.94 |
76.23 |
70.28 |
69.00 |
77.25 |
78.16 |
Weight harvest (kg) |
137.64 |
195.8 |
363.8 |
192.65 |
148 |
241.3 |
Average weight (kg) |
0.083 |
0.086 |
0.162 |
0.090 |
0.060 |
0.098 |
Production (kg/m2) |
0.153 |
0.218 |
0.404 |
0.214 |
0.164 |
0.268 |
Table 1 Survival (%) and production (g/m2) of marron fed basal and probiotic diets
The water temperature in each pond fluctuated daily, but greater water temperature (oC) fluctuation occurred in November (Figure 5 &6). The highest water temperature in marron ponds in average was observed in January and February with an average of 25.61oC to 25.84oC, whereas the lowest (13.05±1.2oC) was observed in July. In addition, a very extreme temperature fluctuation was detected on 21st November where the temperature at night (2400 hours) ranged between 12.7oC to 13.9oC but during the day time (1200 hours) jumped to 36.3oC to 52.7oC which caused high mortality especially in the basal diet fed marron ponds.
In aquaculture, the higher immune status of an animal is crucial as the animal is exposed to a series of stress conditions including the natural rhythms of the environment17,33and the link between stress and higher susceptibility to diseases is conclusive.33Therefore, the improved immunity of the aquatic animal1,4,34 is particularly important to reduce mortalities which lead to significant economic losses and to ensure a profitable aquaculture operation.35 The present in vivo study suggested that customized probiotic B. mycoides significantly improved marron immunity and health status (THC, HSi, intestinal bacteria population and GPx enzyme activity), which in turn improved the survival rate of marron.
The THC of marron fed B. mycoides supplemented diets improved significantly showing that the probiotic B. mycoides remained effective during the entire feeding trial and was able to play a crucial role in marron immunity. It has been proposed by most authors that the marron origin probiotic candidate is preferred as its efficacy is likely to be highest in the host and environment from where it has been isolated.7,19 Improved THC by feeding probiotic diets has also been observed in many crustaceans such as tiger shrimp Penaeusmonodon23 western king prawns P.latisulcatus),36 shrimpsP. japonicus37 and Litopenaeusvannamei.38Moreover, the haemocytes have been successfully used as an immune indicators in various crustacean such as shrimps39,40 lobsters,29,41,42crabs42and crayfishes43,44 including marron46,47 as the haemocytes are involved in various defence mechanisms including recognition, phagocytosis, encapsulation, storage and release of the proPO system and cytotoxicity.48,49,50
It has been documented that the hepatopancreas of crustacean is not just an organ responsible for metabolism but it is an integrated part of immunity.51 The hepatopancreas is an important organ for absorption and storage of large amounts of energy particularly lipids and can synthesize digestive enzymes for food digestion.52It has also been used as an indicator of the marrons’ condition.30,31As the hepatopancreas also serves as source of various enzymes, the larger hepatopancreas of crayfish could be an indicator of greater digestive enzyme activities.53 In the present study, B. mycoides significantly improved HSi of marron compared to HSi of basal diet fed marron both on day 90th and day 160th which suggested that a supplemented probiotic in the marron diet was able to improve the metabolism and energy availability for the animals. These results are in line with Tapia-Paniagua et al.33 who suggested that probiotics increases energy availability of animals and thus improve stress tolerance.
In addition to hepatopancreas, the intestinal bacteria population also plays a significant role in metabolism and immunity. The beneficial bacteria not only protect the animal from the pathogen invasion, but also reflect the nutritional status of the animal.54,55,56 The present study demonstrated that B. mycoides significantly improved the intestinal bacteria population of the marron and also that there was an increase in bacteria population both in basal and probiotic diets fed marron as the marron size increased on day 160th. Ringøet al.57suggested that there is a progressive increase of intestinal bacteria population of small intestines to larger intestines of aquatic animals. Modulation of the intestinal bacteria population have also been demonstrated in many groups of aquatic animals such as Atlantic cod58 Mediterranean teleosts59and Salmonids.2
GPx is another immune parameter of marron, which was improved by feeding with a probiotic supplemented diet. The GPx of marron fed with a probiotic diet was significantly higher than the GPx of basal diet fed marron. Our previous study also revealed a progressive increase of GPxtail muscle tissue of marron with time from one to four weeks feeding. Improved antioxidant enzyme activity by feeding with probiotics have been detected in shrimp Litopenaeusstylirostris.60GPx enzyme activity plays a crucial role in maintaining cellular homeostasis of crayfish 61protects the body from oxidation by free radicals,62 which can cause cellular damage and oxidative stress.63 The GPx activity has also been detected higher in haemocyte of marron.64In addition, probiotics especially lactic acid bacteria exhibit various antioxidant activity which is capable of limiting excessive amounts of reactive radicals in vivo and thus potentially contributes in preventing and controlling several diseases associated with oxidative stress.65
Survival rate and growth are critically important for a profitable aquaculture practices. In the present study, the average survival rate (74.8%) of B. mycoides diet fed marron was significantly higher than the survival rate (66.2 %) of basal diet fed marron as the probiotic was able to improve immunity and stress tolerance of marron when the culture condition extreme. An extreme fluctuation of water temperature on the 21st of November ranged between 12.78oC to 13.94oC at night (2400 hours) and between 36.29oC to 52.72oC during the day time (1200 hours) in the marron ponds triggered high marron mortality on that day and the following days. However, higher immune status particularly the HSi of probiotic fed marron suggested the animals were more adaptable to this chronic environmental stress situation which resulted in a higher survival rate compared to the basal diet fed marron. Jussila et al.46 observed a decreased HSi of marron during a post simulated transport stress test, which suggested that high energy utilization (hepatopancreas) induced by this stress conditions.66In addition, high mortality in basal diet marron pond resulted in more food available for survive marron, thus.
Though immunity and the health status of marron given a probiotic fed diet were significantly higher than basal diet fed marron as described above, the average pond production was still relatively low. This could be partly attributed to a broad spectrum of juvenile size and/quality and their sources used at the initial stocking of the experimental ponds. The basal diet fed ponds were initially stocked with the juveniles produced from the same experimental ponds, whereas most of the juveniles for the probiotic fed marron ponds were obtained from the non-experimental ponds and out sourced and were relatively smaller in size than the juvenile sizes of basal diet fed marron. Lack of juvenile’s sources at initial stocking time contributed to the relatively larger variations in sizes and quality. Therefore, further study is required by using only one source of equal sized juveniles as an initial stocking to evaluate the performance of probiotic diets on marron in commercial marron farms. A comparable size of animal from treatments groups is recommended by some authors when measuring a particular parameters as several parameters vary greatly according to animal size or organs such as bacteria density and microvilli of similar size GIT.55,67
In summary, the customized marron origin probiotic B. mycoides worked effectively in vivo (commercial marron farm) as indicated by a significant improvement of marron immunity and health status (THC, GPx enzyme activity, intestinal bacteria population and HSi) which in turn enhanced survival rates when compared to basal diet fed marron.
None.
None.
The author declares that there are no conflicts of interest.

©2019 Ambas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.