Journal of
eISSN: 2378-3184


Short Communication Volume 5 Issue 5
1Departamento de Pesquer
2Departamento de Conservaci
3Departamento de Ciencias de la Sustentabilidad, El Colegio de la Frontera Sur, Mexico
Correspondence: Adri, Tel 01-612-1225366
Received: April 27, 2017 | Published: May 11, 2017
Citation: González-Acosta AF, Rodiles-Hernández R, González-Diaz AA, Mendoza-Carranza M (2017) Notes on the Presence of Mustelus sinusmexicanus and Hexanchus nakamurai (Chondrichthyes: Elasmobranchii) in Mexican waters. J Aquac Mar Biol 5(5): 00133 DOI: 10.15406/jamb.2017.05.00133
The taxonomy and distribution of 2 deep water sharks, Mustelus sinusmexicanus and Hexanchus nakamurai, is discussed here on the basis of 3 specimens from the Gulf of Mexico. Both species aredistributed in the area; however, they are rarely found in ichthyological collections as they are seldomcaught and due to the challenge of curating such large specimens. Thus, this is the first time that GulfSmooth hound and Bigeye Sixgill shark specimens have been curated in a Mexican ichthyologicalcollection, confirming its presence in México.
Keywords:New documented records, Hexanchidae, Triakidae, Mexican sharks, Mustelus sinusmexicanus, Hexanchus nakamurai
Selachii comprise a taxon of particular interest for their unique biology, ecology, and behavior; but, intensive fisheries have been reduced dramatically their abundances.1 Thus, it is imperative that we taxonomically verify any reports and curate samples of the specimens in the corresponding reference collections. In Mexico, Selachii or sharks are represented by 109 species, which in turn constitute 30% of the world’s species richness.2
In this study, we present information regarding the taxonomic characteristics and distribution of Gulf Smooth hound (Triakidae) and Bigeye Sixgill sharks (Hexanchidae) based on the examination of three specimens from the Gulf of Mexico. While both species are distributed in the area, very few are included in reference collections3,4 this is due to the rarity of capturing them and the difficulty of curating such large samples.
Three specimens of the 2 species reported here were caught by the commercial fishery using bottom long lines near the Campeche Bank in Mexico. These specimens were deposited and cataloged in the ichthyology collection at El Colegio de la Frontera Sur (ECOSC). The specimens were identified following the relevant literature.2,5-11 Additionally, biometrics were recorded for each specimen which follow to Ebert et al.9 and Heemstra10 with some modifications and additions based on the morphology of the organisms examined (Table 1). The nomenclature and taxonomic arrangement are after Page et al.12
|
MSM |
MSM |
HN |
|
|
Biometrics (mm) |
ECOSC 7471 |
ECOSC 7472 |
ECOSC 7414 |
|
Total length |
800 |
1092 |
896 |
|
Pre-caudal length |
614.0 (76.7) |
935.0 (85.6) |
570.0 (65.6) |
|
Pre-narinal length |
32.0 (4.0) |
52.0 (4.7) |
13.0 (1.5) |
|
Pre-oral length |
58.0 (7.2) |
68.0 (6.2) |
51.0 (5.8) |
|
Pre-orbital length |
65.0 (8.1) |
76.0 (6.9) |
34.0 (3.8) |
|
Pre-spiracle length |
40.0 (5.0) |
55.0 (5.0) |
96.0 (10.9) |
|
Pre-gill length |
145.0 (18.1) |
167.0 (15.3) |
123.0 (14.0) |
|
Pre-pectoral length |
160.0 (20.0) |
210.0 (19.2) |
146.0 (16.6) |
|
Pre-pelvic length |
345.0 (43.1) |
490.0 (44.8) |
341.0 (38.9) |
|
Snout-ventral length |
382.0 (47.7) |
475.0 (43.5) |
366.0 (41.8) |
|
Pre-dorsal length |
200.0 (25.0) |
307.0 (28.1) |
420.0 (47.9) |
|
Dorsal-caudal space |
298.0 (37.2) |
453.0 (41.5) |
99.0 (11.3) |
|
Pre-anal length |
525.0 (65.6) |
705.0 (64.5) |
468.0 (53.4) |
|
Pectoral-pelvic space |
168.0 (21.0) |
310.0(28.3) |
143.0 (16.3) |
|
Pelvic-anal space |
151.0 (18.9) |
169.0 (15.5) |
62.0 (7.1) |
|
Anal-caudal space |
80.0 (10.0) |
150.0 (13.7) |
72.0 (8.2) |
|
Pelvic-caudal length |
274.0 (34.2) |
319.0 (29.2) |
68.0 (7.7) |
|
Eye length |
26.0 (3.2) |
28.0 (2.6) |
35.0 (3.9) |
|
Eye height |
12.0 (1.5) |
14.0 (1.3) |
1.8 (0.16) |
|
Inter orbital length |
58.0 (7.2) |
73.0 (6.7) |
72.0 (8.2) |
|
Nostril width |
13.0 (1.6) |
17.0 (1.5) |
8.0 (0.9) |
|
Internarinal width |
17.0 (2.1) |
32.0 (2.9) |
36.0 (4.1) |
|
Anterior nasal fold |
5.0 (0.6) |
7.0 (0.6) |
7.0 (0.8) |
|
Spiracle length |
5.0 (0.6) |
7.0 (0.6) |
3.0 (0.3) |
|
Eye-spiracle length |
7.0 (0.9) |
8.0 (0.7) |
37.0 (4.2) |
|
Mouth length |
57.0 (7.1) |
85.0 (7.8) |
103.0 (11.7) |
|
Mouth width |
44.0 (5.5) |
60.0 (5.5) |
90.0 (10.3) |
|
Upper labial furrow |
16.0 (2.0) |
18.0 (1.6) |
50.0 (5.7) |
|
Lower labial furrow |
13.0 (1.6) |
15.0 (1.4) |
21.0 (2.4) |
|
First gill arch height |
25.0 (3.1) |
41.0 (3.7) |
85.0 (9.7) |
|
Second gill arch height |
27.0 (3.4) |
45.0 (4.1) |
75.0 (8.5) |
|
Third gill arch height |
25.0 (3.1) |
37.0 (3.4) |
63.0 (7.2) |
|
Fourth gill arch height |
28.0 (3.5) |
35.0 (3.2) |
61.0 (6.9) |
|
Fifth gill arch height |
17.0 (2.1) |
22.0 (2.0) |
58.0 (6.6) |
|
Sixth gill arch height |
- |
- |
35.0 (3.9) |
|
Head height |
90.0 (11.2) |
140.0 (12.8) |
77.0 (8.8) |
|
Head width |
150.0 (18.7) |
190.0 (17.4) |
94.0 (10.7) |
|
Trunk height |
65.0 (8.1) |
85.0 (7.8) |
87.0 (9.9) |
|
Trunk width |
73.0 (9.1) |
113.0 (10.3) |
78.0 (8.9) |
|
Caudal peduncle height |
22.0 (2.7) |
30.0 (2.7) |
37.0 (4.2) |
|
Caudal peduncle width |
12.0 (1.5) |
18.0 (1.6) |
24.0 (2.7) |
|
Pectoral fin length |
72.0 (9.0) |
159.0 (14.6) |
105.0 (11.9) |
|
Anterior margin of pectoral fin |
115.0 (14.4) |
170.0 (15.6) |
107.0 (12.2) |
|
Pectoral fin base length |
35.0 (4.4) |
47.0 (4.3) |
44.0 (5.0) |
|
Pectoral fin height |
109.0 (13.6) |
144.0 (13.2) |
82.0 (9.3) |
|
Pectoral fin inner margin |
60.0 (7.5) |
75.0 (6.8) |
49.0 (5.6) |
|
Posterior margin of pectoral fin |
100.0 (12.5) |
125.0 (11.4) |
88.0 (10.0) |
|
Pelvic fin length |
74.0 (9.2) |
103.0 (9.4) |
81.0 (9.2) |
|
Anterior margin of pelvic fin |
67.0 (8.4) |
75.0 (6.8) |
40.0 (4.5) |
|
Pelvic fin base length |
48.0 (6.0) |
50.0 (4.6) |
56.0 (6.4) |
|
Pelvic fin height |
47.0 (5.9) |
47.0 (4.3) |
25.0 (2.8) |
|
Pelvic fin inner margin |
40.0 (5.0) |
55.0 (5.0) |
23.0 (2.6) |
|
Posterior margin of pelvic fin |
53.0 (6.6) |
73.0 (6.7) |
56.0 (6.4) |
|
Outer clasper length |
65.0 (8.1) |
- |
27.0 (2.9) |
|
Inner clasper length |
78.0 (9.7) |
- |
28.0 (3.2) |
|
Clasper base length |
10.0 (1.2) |
- |
8.0 (0.9) |
|
First dorsal fin length |
115.0 (14.4) |
179.0 (16.4) |
65.0 (7.4) |
|
Anterior margin of first dorsal fin |
97.0 (12.1) |
163.0 (14.9) |
50.0 (5.7) |
|
First dorsal fin base length |
87.0 (10.8) |
130.0 (11.9) |
49.0 (5.6) |
|
First dorsal fin height |
64.0 (8.0) |
103.0 (9.4) |
26.0 (2.9) |
|
Inner margin of first dorsal fin |
50.0 (4.6) |
50.0 (4.6) |
18.0 (2.0) |
|
Posterior margin of first dorsal fin |
90.0 (8.2) |
149.0 (13.6) |
45.0 (5.1) |
|
Second dorsal fin length |
86.0 (10.7) |
116.0 (10.6) |
- |
|
Anterior margin of second dorsal fin |
77.0 (9.6) |
101.0 (9.2) |
- |
|
Second dorsal fin base length |
56.0 (7.0) |
85.0 (7.8) |
- |
|
Second dorsal fin height |
49.0 (6.1) |
64.0 (5.8) |
- |
|
Inner margin of second dorsal fin |
33.0 (4.1) |
73.0 (6.7) |
- |
|
Posterior margin of second dorsal fin |
64.0 (8.0) |
78.0 (7.1) |
- |
|
Anal fin length |
56.0 (7.0) |
80.0 (7.3) |
53.0 (6.0) |
|
Anterior margin of anal fin |
49.0 (6.1) |
68.0 (6.2) |
23.0 (2.6) |
|
Anal fin base length |
40.0 (5.0) |
51.0 (4.7) |
37.0 (4.2) |
|
Anal fin height |
25.0 (3.1) |
45.0 (4.1) |
14.0 (1.6) |
|
Inner margin of anal fin |
20.0 (2.5) |
33.0 (3.0) |
13.0 (1.5) |
|
Posterior margin of anal fin |
22.0 (2.7) |
47.0 (4.3) |
27.0 (3.1) |
|
Dorsal margin of caudal fin |
422.0 (52.7) |
614.0 (56.2) |
282.0 (31.0) |
|
Pre-ventral caudal fin margin |
73.0 (9.1) |
82.0 (7.5) |
63.0 (7.2) |
|
Lower post-ventral caudal fin margin |
27.0 (3.4) |
33.0 (3.0) |
34.0 (3.8) |
|
Upper post-ventral caudal fin margin |
77.0 (9.6) |
85.0 (7.8) |
160.0 (18.2) |
|
Sub-terminal caudal fin margin |
27.0 (3.4) |
34.0 (3.1) |
44.0 (5.0) |
|
Terminal caudal fin margin |
56.0 (7.0) |
67.0 (21.8) |
28.0 (3.2) |
|
Terminal caudal lobe length |
172.0 (21.5) |
254.0(23.3) |
64.0 (7.3) |
|
Caudal fork length |
63.0 (7.9) |
65.0 (5.9) |
60.0 (6.8) |
|
Distance between the origin of the first dorsal fin and the origin of the anal fin |
307.0 (38.4) |
486.0 (44.5) |
46.0 (5.2) |
|
Distance between the origin of the first dorsal fin and the origin of the anal fin |
255.0 (31.9) |
380.0 (34.8) |
34.0 (3.9) |
|
Distance between the origin of the second dorsal fin and the origin of the anal fin |
48.0 (6.0) |
90.0 (34.8) |
- |
|
Distance between the origin of the second dorsal fin and the insertion point of the anal fin |
25.0 (3.1) |
54.0 (8.2) |
- |
|
Caudal width space |
22.0 (2.7) |
28.0 (2.6) |
33.0 (3.7) |
|
Caudal fork width |
22.0 (2.7) |
28.0 (2.6) |
33.0 (3.7) |
|
Sex |
Mature male |
Female |
Immature male |
|
Weight (g) |
1,100 |
3,500 |
1,560 |
|
Gastric contents |
Eviscerated |
Eviscerated |
Empty |
Table 1 Morphometric data for Mustelus sinusmexicanus [MSM] and Hexanchus nakamurai [HN] form Mexican waters. Boldface measurements are expressed as percentages of total length.
We identified a total of 2 genera corresponding to an equal number of families and orders, which were described as follows:
Taxonomy summary
Class: Chondrichthyes
Subclass: Elasmobranchii
Order: Carcharhiniformes
Family: Triakidae
Genus Mustelus Linck 1790
The genus Mustelus includes 27 species distributed in tropical, subtropical, and temperate climates of the world’s oceans,5,7,8,9 representing 33.3% (nine) of the species in Mexican waters.2,13 Mustelus sinusmexicanus Heemstra, 1997(Figure 1,Table 1).
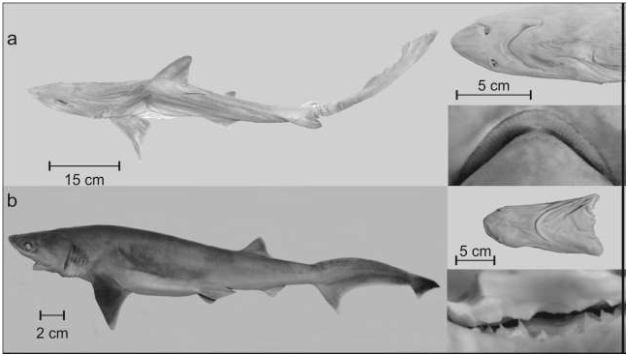
Figure 1 a) Mustelus sinusmexicanus, ECO-SC 7471-1 (male specimen, 800 mm TL, 1,100 g);
b) Hexanchus nakamurai, ECO-SC 7414 (male specimen, 896 mm TL, 1,560 g) from the Gulf of Campeche,
Mexico.
Order: Hexanchiformes
Family: Hexanchidae
Genus Hexanchus Rafinesque 1810
The genus Hexanchus is represented by 2 species with a circumglobal distribution,5,7,8,9 in Mexico’s Atlantic and Pacific watersheds.2,14 Hexanchus nakamurai Teng 1962 (Figure 1b; Table 1)
Due to our limited knowledge of the biology and ecology of these species, they have been assigned a conservation status of “data deficient” [sic] in the IUCN Red List of Threatened Species.15,26 Therefore, it is necessary to conduct basic studies of their population dynamics and other aspects of their basic biology in order to more accurately determine the current conservation status of their populations.5,11
In recent years, there has been an increase in the number of studies reporting new species of sharks,13 including new records of their presence and extensions to their distribution in Mexican waters.1 This situation highlights the fact that we have yet to fully explore the entirety of the country’s cartilaginous fish, making further studies focusing on these species imperative.
We thank the crew of the fishing boat, La Perla del Golfo, and the artisanal fishery based out of the port of San Pedro for capturing these specimens. This project was partially funded by Grant No. 120925 awarded jointly by El Consejo Nacional de Ciencia y Tecnología (CONACyT) and the Tabasco State Government. AFGA is grateful for funding from the SNI-CONACyT, and the EDI and COFAA-IPN Programs. K Sullivan edited the English manuscript.
None.

©2017 González-Acosta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.