Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 6
1Department of Marine Science, Mawlamyine University, Myanmar
2Marine Science Association, Myanmar
Correspondence: Soe Pa Pa Kyaw, Lecturer, Department of Marine Science, Mawlamyine University, Myanmar
Received: November 03, 2019 | Published: November 12, 2019
Citation: Kyaw SPP, Soe-Htun U. Morphology and distribution of Laurencia sp.1 (Ceramiales, Rhodophyta) from Myanmar. J Aquac Mar Biol. 2019;8(6):190-196. DOI: 10.15406/jamb.2019.08.00261
The plants of Laurencia sp. 1 were collected from Nyaw Byin (Lat. 13˚40′N, Long. 98˚00′E) to Sittwe (Lat. 20˚08′ N, Long. 92˚54′E) of Myanmar from 1987 to 2019. Laurencia sp.1 was characterized by the absence of corps en cerise within each superficial cortical cell, and lenticular thickenings in the walls of medullary cells, and the presence of secondary pit-connections between cortical cells, four pericentral cells per axial segment and tetrasporangia formed in the apical portion of the lateral axis with a parallel arrangement and cystocarps with one or more ostioles, based on the external and internal morphologies of both the vegetative and reproductive structures. The distribution and some ecological notes along with potential uses of this species were briefly described.
Keywords: ceramiales, distribution, Laurencia sp.1, morphology, Myanmar, Rhodophyta, taxonomy
The genus Laurencia was established by Lamouroux in 1813. According to Guiry and Guiry,1 the genus Laurencia comprises about 145 species. This genus is widely distributed in temperate and tropical seas of the world.
In Myanmar, Kyi Win2 and Kyaw Soe and Kyi Win3 reported four species of Laurencia, such as L. obtusa Hudson, L. platyclada Boergesen, L. penniculata (Agardh) J. Agardh, and L. papillosa (Forsskal) Greville. Soe-Htun4 accounted that three species of Laurencia, viz., L. obtusa Hudson, L. papillosa (Forsskal) Greville and L. platyclada Boergesen from three coastal regions of Myanmar. Subsequently, Soe-Htun et al.5,6 recorded six species of Laurencia, viz., L. composita Yamada, L. pinnata Yamada, L. intricata Lamouroux, Chondrophycus papillosa (C. Agardh) Garbary and Harper (L. papillosa (C. Agardh) Greville), C. intermedius (Yamada) Garbary and Harper (L. intermedia Yamada), and C. ceylanicus (J. Agardh) Wynne, Serio, Cormaci and Funari (L. ceylanica J. Agardh). Very recently, Soe-Htun et al.7 reported two species of Laurencia, viz., C. intermedius (Yamada) Garbary and Harper (L.intermedia Yamada), and C. ceylanicus (J. Agardh) Wynne, Serio, Cormaci and Funari (L. ceylanica J. Agardh) from Gwa coastal areas. Moreover, Mya Kyawt Wai et al.8 also recorded three species of Laurencia, viz., C. intermedius (Yamada) Garbary and Harper (L. intermedia Yamada), C. ceylanicus (J. Agardh) Wynne, Serio, Cormaci and Funari (L. ceylanica J. Agardh), and Chondrophycus papillosa (C. Agardh) Garbary and Harper (L. papillosa (C. Agardh) Greville) from Mazin coastal areas of the Rakhine State.
Soe-Htun et al.5,6, Hlaing Hlaing Htoon and Soe-Htun9, Sein Moh Moh Khaing and Soe-Htun10 and Thet Htwe Aung11 identified the only one species of Laurencia as L. intricata Lamouroux. In the present study, L. intricata Lamouroux is designated as Laurencia sp.1 according to the literature available at hand. Laurencia sp. 1 has been identified in the present study, based on the specimens collected from the Myanmar Coastal Zones from 1978 to 2019. The objectives of this study are to investigate detailed morphological structures used for identification in the taxonomy, and to know distribution and some ecological features.
Fresh and living plants of the genus Laurencia were collected from the mangrove swamps and rocky shores in the upper intertidal zone of three Coastal Zones of Myanmar, Taninthayi Coastal Zone, Deltaic Coastal Zone and Rakhine Coastal Zone (Figures 1-19) from 2009 to 2019, preserved with 4% formalin in seawater and then prepared as herbarium sheets. Furthermore, herbarium sheets and liquid-preserved specimens of the genus Laurencia collected from the upper intertidal zone along the Myanmar coastal areas during 1987-2019 and deposited in the Herbarium of Department of Marine Science, Mawlamyine University, Myanmar (MMB) were also used for detailed investigations emphasized on the vegetative and reproductive structures in the present study.
The fragments of plant were squashed on microscope slides, and stained with aniline blue (0.5g water soluble aniline blue in 100ml distilled water and 5ml conc. Acetic acid) and acid fuchsin (1% solution in distilled water and 70% alcohol) for detailed observations. The sections were prepared by hand using razor blades. Microscopic measurements were recorded in micrometer (µm) using the ocular meter. External and internal morphological structures were photographed with a Panasonic (Lumix) DMC-TZ 15 digital camera and processed using Adobe Photoshop CS. The distribution, ecological notes and associated algae of this species were also recorded. The classification system of Laurencia sp. 1 basically follows that of Saito,12,13 Saito and Womersley14 and Guiry and Guiry.1
A classification system of the Laurencia sp.1
Phylum: Rhodophyta
Class: Florideophyceae
Order: Ceramiales
Family: Rhodomelaceae
Genus: Laurencia Lamouroux
Species: Laurencia sp. 1

Figures 1-9 Morphology and anatomy of Laurencia sp.1 (Living material): (1) Habit of a plant (MMB 10330); (2) Part of a sterile plant (MMB 10556); (3) Apex of a branch with apical depression (MMB 10558); (4) Surface view of main branch, showing slightly elongated cortical cells (MMB 10559); (5) Secondary discoid holdfast (arrowhead) with stoloniferous branch (arrow) (MMB 10561); (6) Trichoblast (arrow) arising from growing apex (MMB 10562); (7) Cross section of main branch (MMB 10563); (8) Cross section of main branch showing a secondary pit connections (arrowheads) between cortical cells (MMB 10358); (9) Cross section of main branch showing central (c) and five pericentral (p) cells (MMB 10360).
Ecological notes: Plants grow on mud flat or on rocks covered with sand in the upper to lower intertidal zones. This species attached to the substratum by stoloniferous holdfast. The vegetative or reproductive plants can be found from February to November. The reproductive plants are abundantly found in August. Associated algal species are Schizothrix sp., Cladophora sp., C. rupestris, C. vagabunda, Chaetomorpha gracilis, C. linum, Dictyota adnata, Gracilaria foliifera, Gelidium crinale, G. arenarium, Catenella nipae, Ceramium sp., Centroceras clavulatum, Caloglossa bengalensis, Polysiphonia subtilissima, Acanthophora spicifera, Bostrychia tenella, B. binderi.
Potential uses: Laurencia sp. 1 (L. intricata) is one of the economically important seaweeds. It has many useful substances, such as protein, lipid, crude fibre, iodine, ash, moisture, sulphate and carbohydrate15.
Distribution of Laurencia sp. 1
Myanmar distribution: Tanintharyi Coastal Zone: Nyaw Byin, Sitaw, Kawdut, Kalegoke, Kayinthaung, Setse, Yathaetaung, Kyaikkhami, Button I.; Deltaic Coastal Zone: No Data; Rakhine Coastal Zone: Mawtin Point, Ngapali, Sittwe (Present study) (Table 1, Figure 19).
Species |
Coastal Zones |
|||||
TCZ |
DCZ |
RCZ |
||||
From |
To |
From |
To |
From |
To |
|
Laurencia |
Nyaw Byin |
Button I |
- |
- |
Mawtin Point |
Sittwe |
Table 1 The distributional range of Laurencia sp.1 along the three Coastal Zones of Myanmar
Abbreviations: RCR, the rakhine coastal region; ACR, the Ayeyawady and the Gulf of Mottama (Martaban) Coastal Region; TCR, the tanintharyi coastal region.
Laurencia sp. 1 collected from Myanmar was seemingly related to L. intricata Lamouroux which had been reported from various localities in tropical to warm temperate regions in the world: Americas16, Canary Islands17, Mediterranean Sea,18 Japan12 and Philippines.19 Its type locality was the Antilles and Cuba. Authentic material of L. intricata Lamouroux had not been available for comparison.
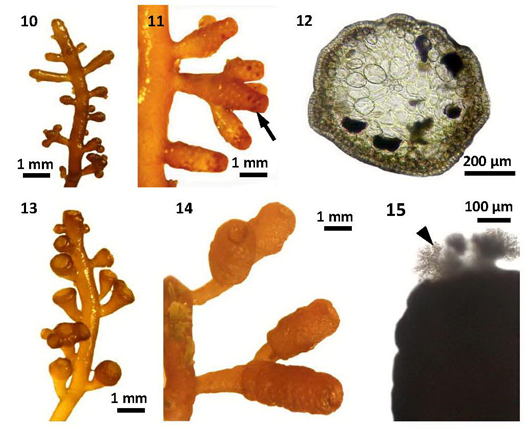
Figures 10-15 Morphology and anatomy of Laurencia sp.1 (Living material): (10) Part of a tetrasporangial plant (MMB 10606); (11) Tetrasporangial stichidia (arrow) on main branch (MMB 10607); (12) Cross section of tetrasporangial stichidium (MMB 10608); (13) Part of a young spermatangial plant (MMB 10614); (14) Part of a mature spermatangial plant (MMB 10616); (15) Spermatangial stichidia with fertile trichoblasts (arrowhead) (MMB 10609).
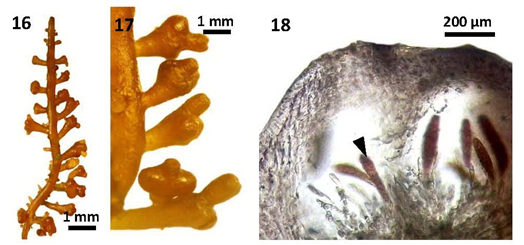
Figures 16-18 Morphology and anatomy of Laurencia sp.1 (Living material): (16) Part of a young carpogonial plant (MMB 10344); (17) Part of a mature carpogonial plant (MMB 10354); (18) Longitudinal section of a mature cystocarp showing elongated carpospore (arrowhead) (MMB 10356).

Figures 19 Local distribution of the Laurencia sp. 1 in Myanmar. 1. Nyaw Byin, 2. Sitaw, 3. Kawdut, 4. Kalagoke, 5. Setse, 6. Kyaikkhami, 7. Button I, 8. Mawtin Point, 9. Ngapali, 10. Sittwe Taninthayi Coastal Zone; Deltaic Coastal Zone; Rakhine Coastal Zone.
Laurencia intricata Lamouroux had been characterized in having matted or tufted, freshly soft thalli with entangled and coalescing, lower branches, the presence of slightly projecting superficial cortical cells, and the absence of both percurrent main axes and lenticular thickenings among the species of the genus Laurencia.12 Furthermore, the species was characterized by the production of four periaxial cells from each vegetative axial segment, and the occurrence of 2-3 corps en cerise per superficial cortical cell (Nam 1990) as cited in Masuda et al.17 These features were based on material from the Pacific. All of these features were found in Myanmar specimens, although superficial cortical cells with single corps en cerise had not been found.
Specimens of L. intricata Lamouroux from Japan12 and Mediterranean Sea,18 having subverticillate or irregular spiral manner branched, were distinct from that of Myanmar specimens. In addition, two to four corps en cerise present within each superficial cortical cell in Mediterranean specimens18 was also distinct from that of Myanmar specimens. The descriptions of male and female plants were not given in Japanese specimens.12 The characters of loosely arranged medullary cells found in Philippines specimens19 of L. intricata Lamouroux differed with those of Myanmar specimens of the present study. Canarian specimens17 of L. intricata Lamouroux were somewhat similar in external appearance to L. sp. 1, but it was distinguished by the presence of two to four corps en cerise within each superficial cortical cell, tetrasporangia formed at the apical portions of main axes, and cystocarps with a single apical ostiole. Moreover, the specimens of L. intricata Lamouroux from Americas coasts16 were closely similar to Myanmar specimens, but differ in their habitat, growing more ample and regular in deep water (to a depth of 36m). In comparison, Laurencia sp. 1 from Myanmar, growing in upper intertidal zone exposed to air during low tide, was characterized in having the absence of corps en cerise within each superficial cortical cell, along with tetrasporangia formed apical portion of the lateral axis, and cystocarps with one or more ostioles (Table 2) (Figures 10-18).
The other closely related species of Laurencia sp. 1 was L. pygmaea Weber-van Bosse (L. decumbens Kutzing). Specimens of L. pygmaea Weber-van Bosse (L. decumbens Kutzing) from Malaysia,20 Australia,21 and Tanzania22 were somewhat similar in their habit, habitat, and diameter of decumbent thallus to Laurencia sp. 1, but it was distinguished by the presence of lenticular thickenings in the walls of medullary cells, single or two corps en cerise within each superficial cortical cell, form and structure of tetrasporangia stichidia and cystocarps. However, in the present specimen, there were no lenticular thickenings in the walls of medullary cells and corps en cerise within each superficial cortical cell, and different type of formation in the development of tetrasporangia stichidia and cystocarps (Table 2). Thus, Laurencia sp. 1 appears to be distinguished from all known intricate species of Laurencia, from available literature in hand.
Sr. no. |
Characters |
Species |
||
Laurencia sp.1 |
Laurencia intricata Lamouroux |
Laurencia pygmaea |
||
|
Vegetative Structures |
|
|
|
1. |
corps en cerise |
absence |
presence |
presence |
2. |
lenticular thickenings |
absence |
- |
presence |
|
|
|
|
|
|
Reproductive Structures |
|
|
|
3. |
Formation of tetrasporangia |
formed apical portion of the lateral axis |
formed at the apical portions of main axes |
- |
4. |
Cystocarps with number of ostioles |
cystocarps with one or more ostioles |
cystocarps with a single apical ostiole |
- |
Table 2 Comparison of the taxonomic features in Laurencia sp. 1 and its other related species
Laurencia sp. 1 distributes in the Tanintharyi Coastal Zone, from the southern limit of Nyaw Byin (Lat. 13˚40′ N, Long. 98˚00′E) to Button I. (Lat. 16˚01′N, Long. 97˚58′E), no data in Deltaic Coastal Zone, and in the Rakhine Coastal Zone, from the southern limit of Mawtin Point (Lat. 15˚58′ N, Long. 94˚14′E) to the northern limit of Sittwe (Lat. 20˚08′ N, Long. 92˚54′E). In general, local distribution range of L. sp. 1 is widely distributed from the southern limit of Nyaw Byin (Lat. 13˚40′ N, Long. 98˚00′ E) of the Tanintharyi Coastal Zone to the northern limit of Sittwe (Lat. 20˚08′N, Long. 92˚54′ E) of the Rakhine Coastal Zone.
In the present study, the specimens were collected from the upper intertidal zone of muddy and rocky areas of Nyaw Byin (Lat. 13˚40′N, Long. 98˚00′E) to Sittwe (Lat. 20˚08′N, Long. 92˚54′E) of Myanmar. The collected specimens were identified as Laurencia sp. 1 based on the morphologies of both vegetative and reproductive structures. Although Laurencia sp.1 should be identified as a new species, it could not be described as of new species due to limited literature available at hand. For this reason, it is tentatively designated as Laurencia sp.1 so far. Local distribution range of L. sp. 1 is widely distributed from the southern limit of Nyaw Byin of the Tanintharyi Coastal Zone to the northern limit of Sittwe of the Rakhine Coastal Zone.
We are indebted to Dr. Aung Myat Kyaw Sein, Rector of Mawlamyine University, Dr. Mie Mie Sein and Dr. San San Aye, Pro-Rectors of Mawlamyine University for their permission. We would like to express Dr. San Tha Tun, Professor and Head of Department of Marine Science, Mawlamyine University, for his valuable suggestions and permission to utilize the departmental facilities. We are indebted to the late Dr. Min-Thein, Director (Retd.), Myanmar Phamaceutical Factory (MPF), Sagaing, Myanmar for literature provided. Thanks are also due to all our respected teachers and colleagues for their encouragement.

©2019 Kyaw, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.