Journal of
eISSN: 2378-3184


Research Article Volume 6 Issue 4
State Key Laboratory of Virology College of Life Sciences Wuhan University China
Correspondence: Xiao Lin Meng State Key Laboratory of Virology College of Life Sciences Wuhan University China, Tel 86-13607192748
Received: September 21, 2017 | Published: September 25, 2017
Citation: Wang N, Meng MX, Wang MN, Meng XL (2017) Immunological Responses of Procambarus Clarkii to MAP30 and its Efficacy to Protect Shrimp against White Spot Syndrome Virus and Vibrio Alginolyticus. J Aquac Mar Biol 6(4): 00160 DOI: 10.15406/jamb.2017.06.00160
Momordica anti-HIV protein 30 (MAP30) is a novel antiviral, antitumor, and antimicrobial protein, and then we revealed that recombinant MAP30 (rMAP30) regulated the activity of immunological factors, phenoloxidase, and superoxide dismutase in hemolymph. The antimicrobial activity of rMAP30 was determined in vitro. Moreover, rMAP30 also facilitated the subsequent clearance of Vibrio alginolyticus in Procambarus clarkii, Challenge experiments with WSSV (100% mortality and 70% mortality) demonstrated significant protection in crayfish with relative survival rate 60% and 85, 5%, respectively. By quantitative RT-PCR analysis, rMAP30 prolonged the survival of WSSV-infected shrimp, and inhibited WSSV replication in Procambarus clarkii. In conclusion, result of this study suggest that rMAP30 may be a candidate for the prevention of WSSV and vibriosis.
Keywords: MAP30, White spot syndrome virus, alginolyticus,P.clarkii, Immunological factors
MAP30, Momordica Anti-HIV Protein 30; rMAP30, Recombinant MAP30; WSSV, White Spot Syndrome Virus; PO, Phenoloxidase; L-dopa; L-3, 4-Dihydroxy-Phenylalanine; SOD, Superoxide Dismutase; P. clarkii, Procambarus Clarkii
White spot syndrome (WSS), caused mainly by the white spot syndrome virus (WSSV).1 is a major disease in crustaceans, particularly for shrimp.2-5 Since its discovery in Taiwan in 1992.6 the virus has spread rapidly causing significant losses to shrimp production almost all over the world.7-11 Shrimp infected with WSSV, principally display white spots or patches embedded in the exoskeleton and epidermis.12 and show a cumulative mortality of upto 100% within 10 days in commercial shrimp farms.13 Previous strategies to prevent and control WSSV, generally included an improvement of environmental conditions, stocking up of specific pathogen-free or pathogen-resistant post-larvae, induction of nonspecific antiviral responses to antiviral drugs or immunostimulants.14-16 neutralization of antibodies.17-20 specific immunization by vaccines.21-26 and suppression of the virus with RNAi technology.27-31
Momordica anti-HIV protein 30 (MAP30) is a novel antiviral, antitumor, and antimicrobial protein from the plant species Momrodica charantia.32 This protein exhibits antiviral activity against the AIDS virus HIV-1.32 the HSV .33 and the HHV8 viruses.34 Momordica anti-HIV protein 30 (MAP30) also exhibits antitumor activity against brain glioblastoma, breast carcinoma, epidermoid carcinoma, liver hepatoma, myeloma, neuroblastoma, prostrate carcinoma, and melanoma as well as AIDS-related lymphoma.35-38 In the current study, we determined the new antiviral activity of the protein MAP30 against WSSV, and the antimicrobial activity against Vibrio alginolyticus in Procambarus clarkii. We also analyzed the immunological parameters in P. clarkii to detect the induction of the humoral immune reaction by MAP30.This protein could be used to develop novel strategies for WSSV and vibrios infection.
Materials
Procambarus clarkii (approximately 20-30 g per sample) was purchased from a local market in Wuhan, Hubei Province, China, maintained in fiberglass tanks at room temperature (25 ± 1°C), and fed with artificial pellet feed, twice a day. Prior to the experiments, the crayfish were screened for the presence of WSSV by PCR and cultured for 2 weeks before treatment, to ensure they were healthy and WSSV-free. WSSV was purified from infected P. clarkii according to a previously described method.39 Vibrio alginolyticus were conserved in our laboratory.
In vivo viral titration
To determine the amount of virus required to achieve mortality greater than 50% and 90% in 10 days, respectively, the virus stock was titrated by in vivo infection experiments. Thirty P. clarkii were injected with 100 µl of different dilutions of WSSV stocks in sterile TN buffer (20 mM Tris-HCl, 0.4 M NaCl, pH 8.5). The negative control was injected with TN buffer alone. Mortality was recorded twice a day, and the dead shrimp were examined by PCR to further confirm infection with WSSV.
Recombinant MAP30 (rMAP30) synthesis
MAP30 was synthesized (Generay Biotech Co., Ltd., China) based on the mature amino acid sequence reported by Lee-Huang (accession number S79450), with an E. coli codon bias. The synthesized MAP30 gene was cloned into pGH-T (Generay Biotech Co., Ltd., China), and the resulting recombinant plasmid was named pGH-MAP30.
Construction of the expression plasmid
Full-length synthetic MAP30 was amplified with polymerase chain reaction (PCR) from pGH-MAP30 with specific primers PF (5’-CGCGGATCCATGGATGTTAACTTCGATTTGTC-3’) and PR (5’-CGCGAGCTCATTCACAACAGATTCCCCAAG-3’). BamHI and SacI sites were designed at the ends of each primer, respectively. The PCR product was digested with BamHI and SacI (New England BioLabsÒ Inc, MA, USA) and then inserted into the pET-28a (+) vector (EMD Millipore, MA, USA). The recombinant plasmid (pET28a-MAP30) was transformed into E. coli BL21 (DE3) (EMD Millipore, MA, USA). Positive transformants were identified by PCR with the PF and PR primers and sequenced in both directions.
Expression and purification of recombinant MAP30 (rMAP30) in E.coli
A single E. coli BL21 (DE3) colony containing pET28a-MAP30 was cultured overnight at 37ºC in LB liquid media containing kanamycin (30 ug/ml). This culture was transferred to 200 ml of fresh media and incubated for 2 h at 37ºC until the OD 600 of the culture reached a value of 0.6. The culture was then induced with 0.5 mM IPTG overnight at 18ºC. MAP30 was purified using Ni-NTA super flow agarose (QIAGEN, Hilden, Germany), according to the manufacturer’s protocol. The eluates were dialyzed with PBS and then concentrated by polyethylene glycol (MW = 20 000) in a bag filter. The protein concentration was determined using the Bradford protein assay. The purified protein was analyzed by 12% SDS-PAGE and confirmed by western blot.
In vivo anti-WSSV assay
The anti-WSSV activity of MAP30 was tested in healthy P. clarkii shrimps challenged with WSSV. 8 groups of healthy shrimps were divided evenly into two sets (4 groups per set and 30 shrimps per group). For each set, the first group was injected intramuscularly with 100 µL sterile TN buffer as a negative control. The second group was injected with WSSV as a positive control, the third group was injected with WSSV pre-incubated with BSA, and the fourth group was injected with WSSV pre-incubated with 200 µg/mL rMAP30 for 20 min at 25ºC (100 µL per shrimp).
The first set of shrimps was challenged with an injection of WSSV with a dilution that produces 50% mortality. The second set of shrimps were injected with WSSV with a dilution that produces 90% mortality. Shrimp mortality was monitored daily for 15 days, and the dead shrimp were examined by PCR to confirm the infection with WSSV. Protection against WSSV, after the challenge, was calculated as the relative percent survival (RPS). RPS = (1 – mortality of test group/mortality of control group) × 100.
WSSV quantification by qPCR
Three individuals were sampled at 72 h post injection from the 90% mortality set. The 90% mortality set contains the WSSV single-infection group, the WSSV group that is pre-incubated with BSA, and the WSSV group that is pre-incubated with MAP30. The gills were sampled for in vivo WSSV replication analysis. DNA extraction, standard plasmid preparation, and the quantification of WSSV copy number by qPCR were performed as described by Kumar DR et al.39 The qRT-PCR was carried out using the THUNDERBIRD SYBR qPCR Mix (TOYOBO Co. Ltd., Osaka, Japan) and absolute quantification was performed using the ABI7500 machine (Applied Biosystems, Thermo Fisher Scientific Inc., MA, USA). The sequences of the primers used in the qPCR were (F: 5-GCTCCAACACCTCCTCCTTCAC-3; R: 5-CTCGGTCTCAGTGCCAGAGTAGG-3). All the samples were tested in triplicate, and three samples were taken from each group. Distilled water replaced the extracted DNA as a negative control for each new run. The virus concentration was converted to copy number per milligram tissue.
Analysis of immunological parameters
To detect the induction of the humoral immune reaction by MAP30, MAP30 (50 µg in 100 µL of PBS), BSA (50 µg in 100 µL of PBS), and PBS were injected into P. clarkii, respectively. At different time points post injection, the hemolymph samples collected from each group, were analyzed for various immunological parameters.
Phenoloxidase (PO) activity assay
The hemolymph PO activity was detected using L-3, 4-dihydroxy-phenylalanine (L-dopa) dissolved in water according to the procedures outlined by Wang et al.40 Briefly, 2 mg of total hemolymph proteins in 435 µL of Tris-HCl (10 mM, PH 8.0) were mixed with 65 µL of freshly prepared L-dopa (3 mg/mL in water). Following incubation at room temperature for 30 min, 500 µL of 10% (v/v) acetic acid was added to the mixture, and the PO activity was measured by monitoring the absorbance at 490 nm. The PO activity was recorded as ΔA490 per mg total protein per min.
Superoxide dismutase (SOD) activity assay
The SOD activity was determined using an SOD kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Antioxidant capacity in vitro
The antioxidant capacity of MAP30 was evaluated by a method based on the Fenton reaction, described by Halliwell and Gutteridge .41 and Desmarchelier et al. 42 but with modifications. This method detects antioxidants classified as scavengers to hydroxylate free radicals. Briefly, various concentrations of MAP30 (2, 4, 8, 16, 32, 64, 128, 256, 500, 1000, 2000 µg/mL) were added to the reaction medium (Fenton reaction) containing FeSO4 (5 mM), crystal violet (2 mM), H2O2 (100 mM), and Tris-HCl (50 mM, pH 5.5). The mixture was incubated at 25ºC for 10 min, and then measured at 588 nm (As). The control was prepared by the same method, where MAP30 was replaced with BSA. Radical scavenging activity was calculated as follows: RSA(%) = [(As-Ab)/(A0-Ab)]×100, where A0 was the absorbance of the reaction medium before proteins were added, As was the absorbance of the reaction medium after each of the different concentrations of MAP30 was added, and Ab was the absorbance of the reaction medium without Fe2+ and H2O2.
Topological inactivation activity of rMAP30
To identify the topological inactivation activity of rMAP30, the reaction was carried out with a final volume of 10 µL containing either supercoiled Litmus38i or WSSV genome DNA as a substrate. The Litmus38i and the WSSV genome DNA were each incubated with MAP30 at 37ºC for 60 min. In the presence of suitable enzymatic conditions, the products were analyzed using electrophoresis in a 1% agarose gel in Tris-phosphate buffer with Tris-Acetate-EDTA buffer. The gel was stained with ethidium bromide for visual documentation by using short wavelength UV light.
Determination of the antimicrobial effects of rMAP30 in vitro
The antimicrobial test was carried out according to the method developed by Seydim and Sarikus.43 with some modifications. The zone of inhibition assay in the solid media was used to determine the antimicrobial effects of rMAP30 against V. alginolyticus. During the test, paper disks containing either antibiotic or PBS or MAP30, were placed on a lawn of bacteria in plates containing LB agar medium and incubated at 37ºC overnight. The plates were examined to determine the inhibition zone of the disks, and the diameter of the zone was measured.
alginolyticusclearance test in shrimp
The assay was conducted according to previously described methods.44 Briefly, V. alginolyticus (2 x 108 CFU) was incubated with PBS, BSA (200 µg/mL), or MAP30 (200 µg/mL) at 28ºC for 30 min, respectively. After incubation, the samples (100 µL each) were injected into the abdominal segment of the shrimp (divided into 3 groups). At every time point post injection (2 min, 30 min, 1 h, and 2 h), hemolymph (100 µL) was collected from the ventral sinus (3 shrimps for each sample). After serial dilutions with PBS, the sample (50 µL) was plated onto thiosulphate-citrate-bile salts-sucrose agar TCBS plates. The plates were incubated overnight at 37ºC, and the bacterial clones on the plate were counted. The number of bacteria in 100 µL hemolymph was determined. The data were subjected to statistical analysis using an unpaired t-test. Significant differences were accepted at p < 0.05.
Expression and purification of MAP30
Recombinant MAP30 (rMAP30) expression analysis was performed by SDS-PAGE and a band around 30 kDa was observed in the sample collected at 12 h post induction of 0.5 mM IPTG (Figure 1). The corresponding band was not observed in the cell sample obtained prior to induction. The recombinant protein was identified in the soluble form and was present in the supernatant. The protein was purified by the Ni-NTA superflow agarose column from the culture supernatant and was examined by SDS-PAGE. Western blot analysis was used to confirm the expression of the recombinant MAP30 protein.
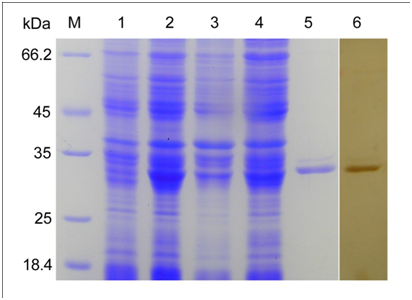
Figure 1 Expression and purification of rMAP30 in E.coli BL21 (DE3). Lane M, protein molecular marker. Lane 1 and 2, total protein from E.coli BL21 (DE3)/PET-28a (+)-MAP30 before and after IPTG induction. Lane 3 and 4, sediment and supernatant after induction, ultrasonication, and centrifugation. Lane 5, purified recombinant MAP30. Lane 6, purified rMAP30 was analyzed by western blotting with anti-MAP30 IgGs.
Anti-WSSV activity in vivo
To determine whether rMAP30 has anti-WSSV activity in shrimps in vivo, the shrimps were intramuscularly injected with sterile TN (negative control), WSSV (positive control), and WSSV pre-incubated with rMAP30 or BSA.
In the first set (70% mortality, WSSV injected), mortality was first observed at 3 days post infection in the positive control group and the BSA-treated WSSV group, while mortality was observed only 3 days later in the rMAP30 group. After 15 days, the group that was treated with rMAP30 showed significantly lower cumulative mortality (9.96%) compared with the positive control (70% mortality) (Figure 2A).
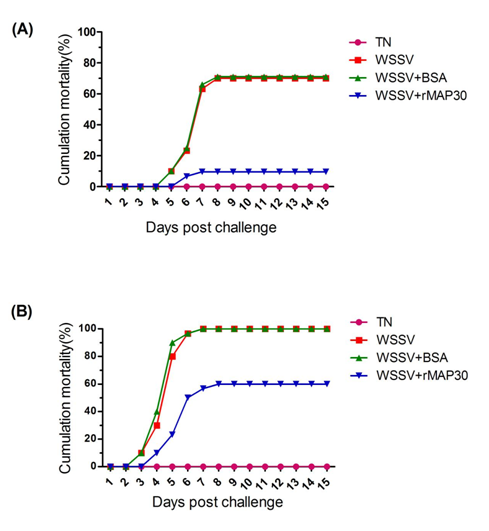
Figure 2 Recombinant MAP30 (rMAP30) anti-WSSV activity in vivo. Shrimps were intramuscularly injected with sterile TN (negative control), WSSV (positive control), or WSSV pre-incubated with rMAP30 or BSA. The mortality was monitored daily for 15 days. (A) 70% mortality; WSSV injected. (B) 100% mortality; WSSV injected.
In the second set, mortality was first observed at 3 days post infection in the positive control group and the BSA-treated WSSV group, while mortality was observed 1 day later in the case of the rMAP30-treated WSSV group. Mortality in the case of rMAP30 was 60%, which was significantly lower than that observed in the positive control group (100% mortality) (Figure 2B).
In the present study, we determined the novel antiviral property of the protein MAP30 with regards to WSSV. For the WSSV challenge, different doses of WSSV were incubated with MAP30 and an intramuscular injection was adopted to ensure an equal challenge pressure. In both the WSSV challenge shrimps, the MAP30-incubated group showed significantly increased survival rates and delayed death from WSSV. The control group experienced high mortality. In the 70% mortality WSSV dose set, the protein showed higher relative survival rates (85.5%) than that in the 90% mortality WSSV dose set (60%).
qPCR assay
The antiviral activity of the MAP30 protein against WSSV was verified by quantification of the viral copy number. The viral DNA levels were quantitated with qRT-PCR analysis. The results showed that the recombinant MAP30 was significantly (p < 0.001) able to inhibit the virus replication of P. clarkii in vivo, by reducing the viral copy number (1.33 ± 0.5×106) compared with the untreated control (5.57 ± 0.2×106). The DNA levels of MAP30-treated shrimps declined and cleared faster than the untreated shrimps (Figure 3). The results showed that MAP30 effectively inhibits WSSV proliferation in vivo.
Immunological assays
To evaluate the humoral immune response induced by rMAP30 in P.clarkii, the hemolymph samples were collected from different groups of shrimp and analyzed for various immunological parameters.
Phenoloxidase (PO) activity
In MAP30-injected shrimp, the PO activity increased significantly at 12 h post treatment when compared with the control group that was injected with PBS (Figure 4A). Phenoloxidase activity reached the highest level at 24 h post treatment. Following 24 h, the PO activity began to decrease gradually and returned to the original levels at 72 h.
Superoxide dismutase (SOD) activity
The SOD activity of MAP30-injected shrimp was significantly decreased compared with that of the negative control group at 2, 6, 12, and 24 hours post-treatment, and a valley was observed at 6 h. The SOD activity gradually increased back to its original level 3 days post administration (Figure 4B).
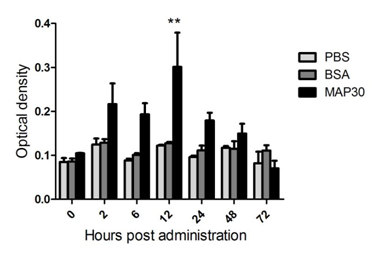
Figure 4 (A) Recombinant MAP30 (rMAP30) leads to PO activation in vivo. (B) Recombinant MAP30 (rMAP30) decreased superoxide dismutase activity in vivo. The error bar represents the standard deviation of mean ± SEM (n = 5). Asterisks or double asterisks indicate significant differences (P < 0.05 and P < 0.01, respectively) compared with the control group that was injected with PBS and BSA.
Prophenol oxidase (ProPO) and antioxidant defense systems are important components of the invertebrate humoral immune response.45 To further investigate the bioactivities of MAP30 in P. clarkii, the phenoloxidase and the SOD activities in the P. clarkii hemolymph were analyzed. After injection of MAP30, the PO activity increased quickly at 2 h. The increase lasts until 72 h and reaches the highest level at 24 h, indicating that proPO was induced by MAP30 in P. clarkii. We inferred that one way MAP30 may take part in WSSV and V. alginolyticus defense is by regulating humoral immune reactions. However, the activity of SOD in the serum of P. clarkii significantly declined following injection of MAP30, but MAP30 exhibited no SOD activity in vitro as well as in previous reports.46 We propose that MAP30 may have an antioxidant capacity, and as expected, the protein was determined by the scavenging activity of hydroxyl radicals. Antioxidants may scavenge free radicals in shrimps. The decline of SOD in shrimp may associate with lower levels of free radicals in shrimps. In fact, during the production and the cleanup of free radicals in homeostasis in vivo, the presence of excessive free radicals can damage the organism.47
Antioxidant capacity in vitro
The antioxidant capacity of rMAP30 in vitro was measured by Fenton reaction that determines the scavenging activity of hydroxyl radicals. As shown in Figure 5, the OH-RSA of MAP30 increased with an increase in concentration, and the scavenging activity at 2 mg/mL was 25%. Thus, MAP30 displayed an antioxidant capacity and a scavenging activity of hydroxyl radicals.
Topological inactivation
To explore how this protein act on virus ,we demonstrate the topological inactivation activity, supercoiled DNA (Litmus38i) and WSSV genome DNA were exposed to rMAP30, and DNA in the absence of rMAP30 was treated as a control. In suitable enzyme digestion conditions, rMAP30 cleaved the supercoiled double-stranded DNA to produce nicked circular or linear DNA. The genome DNA of the virus treated with rMAP30, was degenerated and the band obscured. As shown in Figure 6, rMAP30 exhibited DNase-like activity. Viral genome DNA and rRNA were degenerated after treatment with rMAP30. The DNA topological inactivation ability converts the DNA into topologically inactive forms that interrupt DNA function.48 MAP30 also has an N-glycosidase activity that acts on rRNA in a cell-free system. The ability of MAP30 to act on both DNA and RNA substrates may provide novel mechanisms for its antiviral actions.
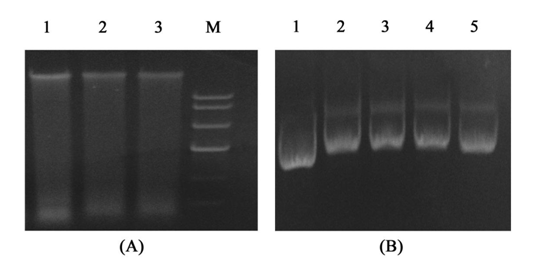
Figure 6 Topological inactivation activities. (A) Destruction of the WSSV genome DNA by rMAP30. Lane 1 – WSSV genome DNA as a control; Lanes 3-4 – the products of DNA with rMAP30; Lane M – DNA ladder; (B) rMAP30 cleaved the super coiled double-stranded DNA. Lane 1 – Litmus38i as a control; Lanes 2-5 – products of Litmus38i DNA treated with different concentrations of rMAP30 (0.8 mg, 0.4 mg, 0.2 mg, 0.1 mg).
Antimicrobial effects of rMAP30
The results of the antimicrobial activity of rMAP30 are shown in Table 1 and Figure 7. Recombinant MAP30 showed antibacterial activity against Vibrio alginolyticus at a concentration of 700μg/ml. The protein showed the effective inhibition towards Vibrio alginolyticus and the inhibition zones were more than 11 mm in diameter. The antimicrobial active principles of rMAP30 are still not clear, and the antimicrobial activation of MAP30 could be explored on other bacteria, it may be tested further to uncover their therapeutic potential in modern and traditional medicine against infectious diseases. It has much work to do explore the antimicrobial effects of rMAP30 in future.
|
Tested Microorganisms |
V. alginolyticus |
|
Zone of Inhibition |
11.10 |
Table 1 Antimicrobial activity of rMAP30 based on a disc diffusion method (Zone of inhibition in mm)
Clearance of V. alginolyticus
To examine whether MAP30 facilitates the subsequent clearance of V .alginolyticus, the shrimps were injected with V .alginolyticus that was pre-incubated with MAP30. Phosphate-buffered saline (PBS) and BSA were used as controls. As shown in Figure 8, V. alginolyticus was rapidly eliminated in all the 3 groups (PBS, BSA, rMAP30), and almost all the bacteria were cleared within 2 h after injection. While bacterial survival in the hemolymph of rMAP30-treated shrimp was significantly lower at 30 min post treatment than in the other 2 groups, this difference decreased over time. However, bacterial counts in the test group were still lower than those in the other groups, suggesting that rMAP30 facilitated the clearance of V .alginolyticus in shrimp. The antimicrobial assay reveals that MAP30 can inhibit the growth of V. alginolyticus in vitro. The clearance of V.alginolyticus in P. clarkii demonstrated that the residual counts of the V. alginolyticus pre-incubate with MAP30 in the P. clarkii haemolymph is significantly lower than that in the control group. This suggests that MAP30 can facilitate the clearance of V. alginolyticus in shrimp.
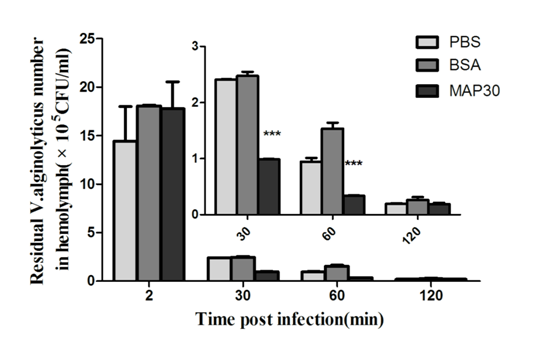
Figure 8 V. alginolyticus clearance in vivo. The shrimps were injected with V. alginolyticus that were incubated with PBS, BSA, or rMAP30, as described in Section 2. The number of V. alginolyticus in 100 µL hemolymph was determined by counting the plates. All the results represent the mean of 3 individual experiments. Asterisk indicates significant differences (P < 0.0001).
Vibrio alginolyticus and WSSV are the most common and serious pathogens that have caused huge economic losses to shrimp aquaculture. V.alginolyticus often co-infects shrimp with WSSV. In the current study, we determined the new antiviral activity of the protein MAP30 with WSSV and the antimicrobial activity towards V.alginolyticus in P. clarkii. MAP30 facilitates the clearance of V. alginolyticus in P. clarkii, and shows antiviral activity against WSSV. The humoral immune reactions of P. clarkii are regulated by MAP30. Although further studies are needed to elucidate the molecular mechanisms, these results suggest that MAP30 may be a candidate for the prevention of WSSV and vibriosis.
The authors are thankful to State Key Laboratory of Virology, College of Life Sciences, Wuhan University and local shrimp farming.
None.

©2017 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.