Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 1
1Departamento de Oceanografia e Limnologia Universidade Federal do Rio Grande do Norte Brazil
2PPG Biologia Estrutural e Funcional Universidade Federal do Rio Grande do Norte Brazil
Correspondence: Sathyabama Chellappa Departamento de Oceanografia e Limnologia Universidade Federal do Rio Grande do Norte Brazil
Received: January 09, 2018 | Published: January 31, 2018
Citation: Nascimento WSD, Silva GMM, Teixeira L, Silva NBD, Chellappa S (2018) Histology of the Digestive Tract of Anablepsoides urophthalmus from Brazilian Oriental Amazonia. J Aquac Mar Biol 7(1): 00181. DOI: 10.15406/jamb.2018.07.00181
This study investigated the anatomy and histology of the digestive tract of the fish Anablepsoides urophthalmus of the family of Rivulidae, captured from the flood plains of northern Brazil. Histology of the digestive tract of this species was investigated, since there is a scarcity of information regarding its digestive biology. This work describes the histological characterization of the digestive tract of A. urophthalmus and correlates the histology of each component with its respective function. This species has only esophagus and intestine and it was possible to identify these components through histological analysis of the mucosa, submucosa, muscular and serous layers. The esophagus is short, with stratified epithelium, cubic with mucous cells and with a muscle layer with two sub-layers of striated muscle fibers. The intestine is coated with simple cylindrical columnar epithelium with microvilli goblet cells. It is divided into anterior, middle and posterior portions, without any folding or glands. The differentiation of regions of the intestine occurs at the level of the intestinal mucosa at the villus and variations of goblet cells.
Keywords: killifish, flood plains, histology, digestive tract, Anablepsoides urophthalmus
The family Rivulidae is one of the largest families of freshwater fishes of the Neotropical region.1 It is a diverse group of annual fish, most popularly known as "killifish", which occurs in seasonal freshwater swamps of tropical and subtropical areas of South America.2 Brazil has the greatest diversity of the family Rivulidae and is represented by 30 genera with approximately 120 fish species.3
Anablepsoides Huber, 1992 is a species-rich genus of Neotropical aplocheiloid killifishes, occurring in northern South America, including the Amazon River basin and in adjacent Caribbean islands.2 The Amazon River basin has the highest diversity both in number of species and in specialized forms. The two groups of fish species, Anablepsoides urophthalmus and Anablepsoides limoncochae are similar, including species of medium size that reach about 40-60 mm, with longitudinal rows of red dots on the flank in males.4
Interpretations of feeding habits based only on analysis of gastrointestinal contents might lead to incorrect conclusions, since the frequency of these items are usually related to their availability in the environment. The digestion rate of the different food items varies, consequently, the presence of food items, which are difficult to be digested, may overestimate their importance in the diet of a given species.5
Morphological and histological studies are very useful for the characterization of the digestive tract, which contributes important information for understanding the feeding habits and digestive physiology of fish.6 Histological analysis of the digestive tract of fish is essential since it provides information which can formulate models which explain the trophic structure of the ecosystems and quantitative knowledge of the biological mechanisms of interaction between species.7
The present work investigated the histology of the digestive tract of Anablepsoides urophthalmus. The histological description of the digestive tract of A. urophthalmusis compared with other teleost fish, to know whether they conform to a standard pattern.
Study site and fish sampling
The Igarapé Fortaleza hydrographic basin, located in the Municipality of Macapá and Santana, in the State of Amapá (Northern Brazil) is composed of the main water channel which is a tributary of the Amazon River and by the floodplain areas. This ecosystem is composed mainly by the Amazon River and the main channel of the Igarapé Fortaleza hydrographic basin. The Amazon River floodplains are fluvial silted systems drained by freshwater and influenced by the high rainfall rates of the Amazonia and by the tides of the Amazon River.8
The sample collection of A. urophthalmus was authorized by ICMBIOMMA (Chico Mendes Institute for Biodiversity Conservation of the Brazilian Ministry of Environment), and SISBIO (Authorization system and Biodiversity information), with the official authorization number of 48725-1. Fish samples were captured from the flood plains located in the Municipality of Santana, AP, Brazil (0°2’5.024”S and 51°9’ 51.34”W), during the period of May to October, 2015. Fish were captured utilizing small hand trawl nets (50x150cm) and sieves (60 x 60cm) of 2mm mesh size. The captured fish were immersed for about 15 minutes in Benzocaine hydrochloride solution at a concentration of 100mg/L,9 according to Resolution No. 714 of June 20, 2002 which deals with euthanasia procedures for animals.
Laboratory procedures
The taxonomic identification of the fish species was confirmed.10 Each fish was dissected to expose the digestive tract, which was removed from the coelomic cavity. Tissue samples were fixed in 10% formalin for 24 hours. After fixation the digestive tract was submitted to microtomy to obtain fragments of the digestive organs. The fragments were submitted to routine histological techniques, such as, dehydration, diaphanization and paraffin embedding. They were micro sectioned at 5μm. Samples were stained with Haematoxylin-eosin (HE)11 and periodic acid Schiff (PAS) reactive method for the identification of neutral mucin and other substances.12 For the slide microphotography procedure, an Olympus BX41 microscope coupled to a Nikon DXM 1200 digital camera was used. The images were processed using Nikon software (Image-Pro Explress) in the Neuroanatomy laboratory of the Federal University of Rio Grande do Norte (UFRN).
Total body length (cm) and the total weight of body (grams) of 20 fishes were measured. Mean total length and weight of the fishes were 3,52cm and 0,45g respectively. The digestive tract of A.urophthalmus is a thin continuous straight tube. As such, it was not possible to distinguish externally anatomical organs, such as, the esophagus, stomach and intestine.
Histology of the esophagus
The esophagus is short with large diameter and has a wall possessing longitudinal folds (Figure 1A). The delicate muscles along with the folds probably permit extensibility to the esophagus during the passage of food. Histology of the esophagus of A.urophthalmus revealed a mucous layer, having predominantly primary folds and few secondary folds, lined by cubic stratified epithelial tissue with mucous cells (Figure 1B) (Figure 1C).
The lamina propria consisted of loose connective tissue, without the presence of glands, but with some striated muscle fibers from the circular muscle layer toward the folds. Mucosal muscle was not present, as such there was no submucosa. The muscle layer was formed by striated muscle fibers (Figure 1D) organized in an internal circular layer and an external longitudinal layer. As it approached the intestine the folds disappeared. The lamina propria presented with predominance of adipose cells (Figure 1D), located on the muscular layer. The last layer was the serosa.
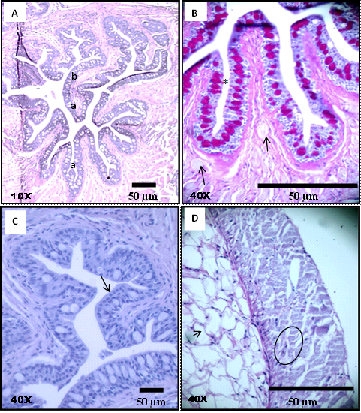
Figure 1 Photomicrographs of histological preparations from a cross-section of the esophagus of Anablepsoidesurophthalmus: A)Mucosa with primary folds; B)Gastric mucosa with cubic stratified epithelium with centralized mucous cells (*) between the epithelial cells and loose connective tissue with some striated muscle fibers (arrow) departing from the muscle layer toward the folds; C. Cubic stratified epithelial cells (arrow); D)Muscle layer with only a layer of striated muscle fibers (circle) and fat cells (arrow).
Histology of the intestine
Analysis of light microscopy permitted differentiation of the layers and cells of the intestinal region. In the anterior part of the intestine, tunica mucosa had villi. The epithelial tissue lining was simple and cylindrical with microvilli, indentations, absorptive cells and goblet cells (Figure 2A1) (Figure 2A2).
The lamina propria is a very thin layer formed by loose connective tissue, without presence of glands and muscular mucosa, hence there was no submucosa. The muscle layer is formed by smooth muscles divided into two layers and with marked myocytes. The mysenteric plexus was found between these two layers. In the mid part of the intestine there was the presence of primary folds, branching to give secondary villi (Figure 2B1 & 2B2). This could be an amplified area of digestion/absorption due to the presence of branched folds, which were frequent when compared to the other regions of the intestine. The other layers were similar to that of the anterior intestine.
The hind region of the intestine was easy to distinguish due to the presence of smaller villi in comparison to the villi present in the anterior and mid intestine. They have the same kind of simple cylindrical covering, with microvilli and plenty of big goblet cells (Figure 2C1) (Figure 2C2). In addition to the previously mentioned difference, a separate layer of loose connective tissue with larger and frequent blood vessels with no glands was also observed. The other layers showed the same pattern as in the earlier mentioned regions.
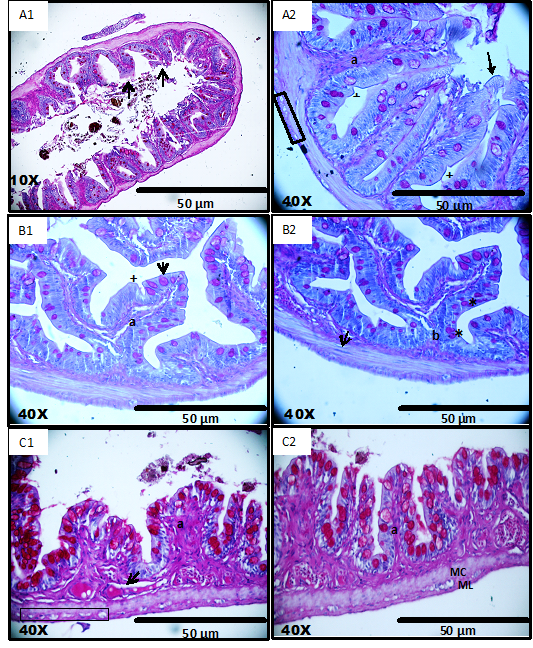
Figure 2 Photomicrographs of histological preparations from cross-sections of the three regions of the intestine of Anablepsoidesurophthalmus: A1 & A2 anterior Intestine: simple cylindrical layer with microvilli (arrow), villi (a) with goblet cells (*), indentations (+), thin lamina propria and muscular layer of smooth muscle fibers divided into two layers and myenteric plexus (rectangle);B1 & B2 middle Intestine: presence of villi(a), primary folds (b) and secondary, simple columnar epithelium with microvilli (arrow), indentations (+) goblet cells in the apical region of tissue and smooth muscle layer divided into two layers and with marked myocytes (arrow); C1 & C2 posterior Intestine: simple cylindrical layer with microvilli and goblet cells in the apical region, thick lamina propria and blood vessels(arrow) and muscular layer divided into two layers (MC- circular and ML- longitudinal) between the myenteric plexus (rectangle).
The distinction between the anterior, middle and posterior regions of the intestine was only possible with light microscopic studies (Figure 3). The microstructure of the intestinal villi presented evaginations of larger mucosa in the anterior and middle regions of the intestine, thus enlarging the contact surface with the food and allowing greater efficiency for digestion and absorption. In the posterior intestine there is a decrease in the height of the mucosa, and the goblet cells were larger and in greater numbers. The muscle layer maintained the same thickness for all intestinal regions.
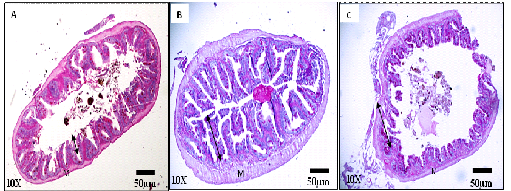
Figure 3 Photomicrographs of histological preparations from cross-sections of anterior (A), middle (B) and posterior (C) regions of the intestine of Anablepsoidesurophthalmus showing the mucosa with the different heights of the intestinal villi (double-headed arrow). Intestinal villi are higher and branched at the middle portion (B) and lower and thicker at the posterior portion (C). The muscle layer (M) with only a muscle fiber striated layer with same size and thickness in all regions.
The short esophagus indicates that the food items are directly taken to the intestine which has no anatomical boundaries. This pattern is similar to that of the lampreys, which are fish without stomach, where the esophagus is directly connected to the intestine.13 In small and omnivorous species such as, Roeboides xenodon, Orthospinus franciscensis14 and also in Pseudoplatystoma corruscans15 the esophagus is short and this portion of the digestive tract does not offer obstacle to the passage of food, similar to the observation in the study species. Similarly, marine carnivorous fish of the family Lutjanidae, such as, Lutjanus synagris, Lutjanus purpureus and Ocyurus chrysurus have short esophagus and this organ is only associated with the passage of food.16
Although epithelial characterization using light microscopy has revealed a stratified cubic epithelium, further detailed studies using scanning electronic microscopy and transmission is necessary since secreting epithelia are usually simple.17
The characteristics of striated muscle layer in A. urophthalmus, indicate that it is possibly involved with the habit of swallowing larger prey, besides giving flexibility. The muscle layer strengthens the wall of the esophagus and protects it from being damaged while swallowing solid materials.18 The freshwater fish, Pelteobagrus fulvidraco is omnivorous in feeding habit, which has a short esophagus with two well developed layers of striated muscles. This arrangement of striated muscle bundles in the esophagus has the capability to reject the passage of a food items which are unpalatable.19 A similar function was observed in Pleuronectes americanus and Pleuronectes ferruginea.20 The striated muscles in the esophagus possibly produce the force needed to push the food to the intestine, under the voluntary control of the fish.
Gastric glands were not observed along the digestive tract of the study species, indicating the absence of stomach. Similarly gastric glands were not found in seahorse, Hippocampus reidi which is considered as an agástric species, where the food passes directly from the esophagus to the intestine.21 The possible cause of the absence of stomach may be related to food items with large proportions of indigestible components such as sand, mud or microphages feeding habits.22,23 Lack of stomach in fish demonstrates its digestive flexibility, since there is no restriction to the type of feeding habit.24
There is no histological demarcation for the regions of the small intestine, which is thick and straight, being similar to other teleosts.25 This was confirmed for the study species and also in the intestine of marbled swamp eel Synbranchus marmoratus which does not present anatomical characteristics into well-defined segments.26
This pattern of organization of simple columnar epithelium throughout the intestine of A. urophthalmus reveals an intensely absorbing surface, which is important for the process of absorption of nutrients.15,27 In Cyclostome fish without stomach, the essential biochemical processes of digestion occur due to enzymatic activity of the pancreas or mucosa of the intestine.28 The absence of stomach in A. urophthalmus seems to be compensated by the straight long intestine. In the piscivorous fish, Tylosurus gavialoides and Strongylura leiura ferox with extremely short intestines, ranging between 0.37 and 0.55, the posterior region of the intestine has a large diameter and length, providing an equivalent function of the stomach, and also in digestion.29
The carnivorous freshwater fish Culteralburnus has a short intestine and its histology reveals distinct characteristics between regions of the intestine which are related to different functions in the digestive physiology of this species. The anterior region of the intestine has longer folds and well developed intestinal microvilli, with large diameter and thick muscles, indicating that this is the principal region for digestion and absorption of food in this species.30 Similarly in many other species of fish without stomach, the anterior intestine was considered the principal region for food digestion and absorption.31 However in A. urophthalmus, anterior and mid intestinal regions promote digestion and absorption. This was also observed in Hypsolebias antenori (Rivulidae), an annual fish from the northeastern Brazil, where the stomach and the intestine are adapted for digestion and absorption of nutrients.32
The digestive tract of this fish does not conform to the standard pattern of teleost fish, due to the absence of a stomach. Out of the characteristic layers of the digestive tract of vertebrates, only tunica mucosa, muscular and serosa were evidenced. Same size of diameter of the digestive tract of the study species, made it difficult at the macroscopic level to differentiate structures that constitute it. The anterior and middle regions of the digestive tract are more active in nutrient absorption.
The authors wish to thank the National Council for Scientific and Technological Development (CNPq) of Brazil for the financial support awarded during the study period.
None.

©2018 Nascimento, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.