Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 1
1Centre of Aquaculture Biotechnology Research Institute for Aquaculture No 1 Vietnam
2Aquaculture Research Sub Institute for North Central ARSINC Research Institute for Aquaculture No 1 Vietnam
3Faculty of Biotechnology Vietnam National University of Agriculture Vietnam
Correspondence: Vu Thi Trang Centre of Aquaculture Biotechnology Research Institute for Aquaculture No 1 Vietnam
Received: January 19, 2018 | Published: February 27, 2018
Citation: Trang VT, Chi T, Thiet CC, et al. Genetic relationship of asiatic hard clam populations collected in northern coastal provinces in vietnam based on mtDNA sequence analysis. J Aquac Mar Biol. 2018;7(1): 00184 DOI: 10.15406/jamb.2018.07.00184
The genetic relationship of some Asiatic hard clam (Meretrix meretrix) based on mtDNA COI sequence analysis was investigated for populations collected in Thai Binh, Nam Dinh, Nghe An provinces in Vietnam. In addition, this research also targets at species identification based on COI sequences. In total of 59 sequences analyzed, 19 sequences belonged to Meretrix meretrix species with Gen Bank accession number DQ399399.1. 17 sequences of M. meretrix were used for genetic relationship analysis among 3 populations. In which, 6 polymorphic sites, 3 parsimony informative sites and 4 haplotypes observed for the COI gene. Moderately genetic population diversity was observed, overall haplotype and nucleotide diversity were 0.476±0.233 and 0.00151±0.00069, respectively. Generally, genetic differentiation (FST) (FST < 0.15) was moderate. The genetic distance was rather low, which ranged from 0.001 (Thai Binh–NgheAn, Thai Binh–Nam Dinh populations) to 0.002 (Nam Dinh – Nghe An populations). The result of haplotype network constructing indicated that populations shared common haplotype and there was no specific isolation of the haplotypes of the populations. Hence, it showed M. meretrix populations had intimate genetic relationship. The result of phylogenic tree indicated that three M. meretrix populations (Thai Binh, Nam Dinh, Nghe An) had a very small or no genetic variation among populations.
Keywords: population, genetic diversity, genetic relationship, meretrixmertrix, phylogenetic analysis
Asiatic hard clams (Meretrix meretrix), genus Meretrix (Veneridae), are commercially important species in coastal areas of South and Southeast Asia.1 In Vietnam, the northern coastal provinces is the main distributor for the total production of this species.2 These clams were considered to be one of the indigenous mollusks in this region. However, recently, in the coastal areas of some Northern provinces, White clams (Meretrix lyrata) or Ben Tre clams were entered from Southern provinces and produced artificially. As the consequences of rapid development, White clams dominate in number compared to indigenous clams with 85-90% of the mollusk yield. This has led to the changes in the structure of coastal organism communities in general and decrease rapidly the resource of M. meretrix in particular.3
There was a considerable number of studies about genetic of M. meretrix in Asia. Chen et al.4 present phylogenetic relationships of the genus Meretrix by using COI gene sequences. Thereafter, Chen et al.5 built phylogenetic tree for 106 individuals belonging to Veneridae family including M. meretrix. In 2011, He et al.6 used clam specimens collected from the coast of Panjin, Liaoning province, China for complete mitochondrial genome sequencing. The results showed that mitochondrial genome sequence of M. meretrix is 19,826 bp in length, containing 37 genes, in which 12 protein-coding genes, 2 ribosomal RNAs, and 23 tRNAs.6 In general, the genetic studies of M. Meretrix in Asia focus primarily on analyzing the genetic relationship of them to closely related species. However, in Vietnam, studies have focused on resource assessment and reproductive biology, meanwhile, research on genetic of Asiatic hard clam has not paid much attention. The understanding of genetic structure and information about M. meretrix genetic diversity is necessary for the conservation, restoration, and development of this clam resource in Northern part of Vietnam.
Mitochondrial DNA (mtDNA) has been widely studied in almost marine and freshwater fish species, mainly for taxonomic and phylogenetic purposes. The advantages of using mtDNA include its simple maternal inheritance, absence of recombination, and high substitution rates.7 The mitochondrial COI gene is often used to distinguish species in animals because of faciliating in amplification by using PCR method and universal primers.8 This sequence of genes is always conserved among individuals in the same species and the rate of mutation is fast enough to distinguish between species with close genetic relationships.9 In this study, the mitochondrial COI gene sequence was used to identify species and genetic relationship analysis in Meretrix genus that were collected in some Northern coastal provinces in Vietnam.
In total, 60 samples were collected in six locations, including HaiPhong (HP), Thai Binh (TB), Nam Dinh (ND), ThanhHoa (TH), NgheAn (NA) and Ha Tinh (HT) with 10 samples per province (Table 1) (Figure 1). The name Asiatic hard clam is called according to the local community (with the Latin name is Meretrix meretrix). Based on morphological characteristics, collected samples were preliminarily identified as belonging to the M. meretrix. They are large clams with thick shell covered by thin, delicate, straw-coloured or grey periostracum, and a greyish-blue or bluish-brown band on its postero-dorsal margin. The length is greater than the height. The muscle tissue 1-2g/sample was cut and preserved in 96% alcohol at 4°C.
|
Geographic populations |
Sample location (longitude and latitude) |
Collection time |
|
(abbreviation used) |
||
|
HP |
HaiPhong (20°51′59″N, 106°40′57″E) |
August, 2017 |
|
TB |
Thai Binh (20°32′20″N, 106°23′40″E) |
August, 2017 |
|
ND |
Nam Dinh (20°25′13″N, 106°10′05″E) |
September, 2017 |
|
TH |
ThanhHoa (20°08′28″N, 105°18′34″E) |
June, 2017 |
|
NA |
NgheAn (19°10′35″N, 104°58′38″E) |
May, 2017 |
|
HT |
Ha Tinh (18°20′28″N, 105°54′26″E) |
June, 2017 |
Table 1 Collection details for Asiatic hard clam samples
DNA extraction, PCR amplification and sequencing
Total DNA of 60 clam samples was extracted according to the alcohol precipitation method [10]. DNA quality was checked by 0.8% agarose gel electrophoresis and the absorbance at 260nm was measured using Nanodrop and cuvette spectrophotometer (NanoDrop™ 2000C) to determine DNA concentration.
The fragments of COI gene of 60 samples were amplified by PCR reaction with primers according to Folmer et al.8 The primer sequence is as follows: Fw – 5’GGTCAACAAATCATAAAGATATTGG3’ and Rw – 5’TAAACTTCAGGGTGACCAAAAAATCA3’. PCR was carried out in a 37μl volume containing 1U/μlTaq DNA polymerase, 100ng/μl template DNA, 10μM each primer (1μl), 5mM (0.5μl) of each dNTPs, 100mM TrisHCl (pH 8.3), 25mM MgCl2 (2.5μl), 500mM KCl (pH 8.3). The PCR was employed with initial denaturation of 2 min at 94oC followed by 30 cycles of denaturation for 30s at 94oC, annealing at 45oC for 45s and an extension of 72oC for 50s. After the completion of 30 cycles, a final extension step of 10 min at 72oC was performed. The PCR product was then kept at 4oC until removed from the machine. The amplified product was tested in 1.5% agarose gel and visualized using the Uvitec system. The appropriate PCR products then were purified and sequenced.
Data analysis
Sequences of COIwaschecked by Finch TV 1.4.0sofware.11 Then, they were aligned and cut into the same length with BioEdit 7.2.512 using Clustal W under default settings. The BioEdit software was also used to check and determine the similarity degree of sequences and to create the consensus sequence of each population. The program DnaSP 5.013 was used to analyze molecular diversity indices including haplotype diversity (Hd), nucleotide diversity (π). Hierarchical analyses of molecular variance (AMOVA) were performed using Arlequin 3.514 to evaluate population structure. Haplotype network was constructed by using Network 4.6.1.15
Analysis of genetic distance between populations was used MEGA 6.0.16 The evolutionary history was inferred using the Neighbor-Joining method.17 The evolutionary distances were computed using the Kimura 2-parameter method18 and are in the units of the number of base substitutions per site. Venerupis/Ruditapes philippinarum (EU266378.1) and Meretrix petachialis (KY318134.1) were used as out group.
The Blast results from National Center for Biotechnology Information (NCBI) showed that in total of 59 samples, there are 19 samples (32.2%) of M. meretrix with 99-100% identity (Table 2).
|
Population |
No. of samples |
No. of analyzed sequences |
No. of M.meretrix species |
Rate (%) |
|
Hai Phong |
10 |
10 |
0 |
0 |
|
Thai Binh |
10 |
10 |
6 |
60 |
|
Nam Dinh |
10 |
10 |
3 |
30 |
|
Thanh Hoa |
10 |
10 |
0 |
0 |
|
Nghe An |
10 |
10 |
8 |
80 |
|
Ha Tinh |
10 |
9 |
2 |
22 |
Table 2 Number of samples belonging M. meretrix species in investigated populations
These results illustrated that species identification by morphology and molecular biology produced different results. By morphology method, 100% of the samples were classified as M. meretrix. However, by molecular biology method, only 32.2% of samples were identified as M. meretrix based on COI sequences. The remaining (67.8%) were identified as M. petechialis. According to Prashad,19 M. meretrix is a species which experienced the greatest variation in the group of bivalves. Because of shades of shells and shell colors, it was wrongly identified with other species.19 The results obtained in this study do not support the present taxonomic status of M. meretrix and M. petechialis, our goal is to analyze the genetic relationship of M.meretrix in different geographic regions of Vietnam. Specially, we focused to analyze genetic relationship of 3 M. meretrix populations in Thai Binh, Nam Dinh, Nghe An provinces.
Genetic relationship between M. meretrix populations
Mitochondrial genetic diversity
The fragments of COI sequences (650 bp) were obtained from 17 clams from the 3 of 6 populations studied (Thai Binh, Nam Dinh, NgheAn) which had more than 2 clams identified as M. meretrix (with GenBank accession numbers is DQ399399.1). In which, 6 polymorphic sites, 3 parsimony informative sites and 4 haplotypes were observed for the COI gene. The results of M. meretrix populations genetic diversity were shown in Figure 2 & Table 3.
|
Sample site |
No. of sequences |
No. of haplotype (h) |
Haplotype diversity |
Nucleotide diversity (π± SD) |
|
|
|
|
(Hd ± SD) |
|
|
Thai Binh |
6 |
2 |
0.333 ± 0.215 |
0.00051 ± 0.00033 |
|
Nam Dinh |
3 |
2 |
0.667 ± 0.314 |
0.00204 ±0.00096 |
|
Nghe An |
8 |
2 |
0.429 ± 0.619 |
0.00197 ± 0.00078 |
|
Total |
17 |
4 |
0.476 ± 0.233 |
0.00151 ± 0.00069 |
Table 3 Mitochondrial genetic diversity of studied M. Meretrix populations
The COI network was radial-like with a number of unique haplotypes closely related to central haplotype. Figure 2 indicated that the haplotype H_1, accounted for 76.47% (13/17), of all 17 individuals; occupied the central position of the network, one step away with other 3 haplotypes. This also suggested that H_1 was the ancestral haplotype and M. meretrix populations had an intimate genetic relationship. Besides, each population had its own haplotype which specific to the population. Hence, there was no population structure or population structure is not clearly established among studied populations the overall haplotype diversity and nucleotide diversitywere 0.476±0.233 and 0.00151±0.00069, respectively. The Hd ranged from 0.333±0.215 (Thai Binh population) to 0.667±0.314 (Nam Dinh population). Nucleotide diversity was highest (0.00204±0.00096) in Nam Dinh population and lowest (0.00051±0.00033) in Thai Binh population. Hd and π values in this study were lower than previous study on Meretrix petechialis and Ruditapes philippinarum using the same method. In fact, these values were 0.9483±0.0054 and 0.03364±0.01638, respectively in Meretrix petechialis collected in the Northwestern Pacific.20 In Manila clam (Ruditapes philippinarum), Hd values were ranged from 0.80 to1.00 while π values fluctuated 0.17–1.08 in populations collected in Asia.21 Grant and Bowen, 1998 pointed out that marine species which experienced rapid expansion following a period of low effective population size often display high haplotype but medium to low nucleotide diversities.22 Genetic diversity can be influenced by a range of factors including sample size, natural selection, mutation rates, gene flow among populations and human factors.23 Previous studies revealed high genetic diversity in marine species including the miiuy croaker (Miichthys miiuy),24 the fat greenling (Hexagrammos otakii),25 and the clam (Macridiscus multifarius).26
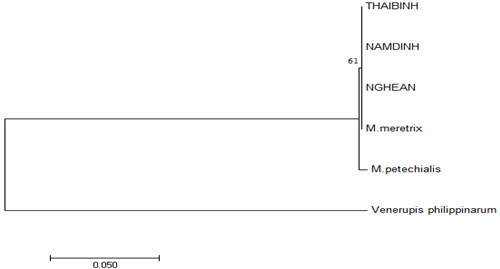
Figure 3 Dendrogram (NJ tree) based on Nei (1978) genetic distance between 3 M. meretrix populations.28
Genetic differentiation and genetic distance
FST values (Table 4) indicated the levels of pair wise genetic differentiation between the 3 populations. The FST values between populations were moderate (FST < 0.15), in which the lowest FST value was observed between the Nam Dinh and NgheAn populations (FST = 0.07996), while the highest FST value was observed between the Nam Dinh and Thai Binh populations (FST = 0.13333).
|
|
Nam Dinh |
Thai Binh |
Nghe An |
|
Nam Dinh |
− |
0.13333* |
0.07996* |
|
Thai Binh |
0.001 |
− |
0.08789* |
|
Nghe An |
0.002 |
0.001 |
− |
Table 4 Genetic differentiation (above) and genetic distance (below) of 3 M. meretrix populations
(*: P value < 0.05)
The genetic differentiation (FST) increased as geographic distance increased was correct for the case of the relationship between Nghe An population and the other, but it was not correct for other relationships. Genetic differentiation is influenced by many factors including habitat differences, historical events and human activities.27
The genetic distance values ranged from 0.001 (Thai Binh – NgheAn, Thai Binh – Nam Dinh) to 0.002 (Nam Dinh – Nghe An). The variation in genetic distance was not correlated with geographic distance. In this study, the geographic distance between NgheAn – Thai Binh was highest, but the genetic differentiation between Nam Dinh – NgheAn were largest.
Hierarchical analysis of AMOVA (Table 5) showed that majority of the molecular variation was distributed within populations (91%) rather than among populations (9%), indicating that the total genetic variation was intrapopulation variation. Therefore, it can be found that the population structure was not clearly established and the genetic diversity was low among studied populations. The FST value was 0.08999 which means there were moderate significant genetic variations among the three M. meretrix populations. Therefore, the use of only COI marker for the mtDNA region had not been polymorphic in this study.
|
Source of variation |
d.f. |
Sum of squares |
Variance components |
Percentage of variation |
Fixation Index FST |
|
Among populations |
2 |
1.451 |
0.04709 |
9 |
0.08999 |
|
Within populations |
14 |
6.667 |
0.47619 |
91 |
|
|
Total |
16 |
8.118 |
0.52328 |
|
|
Table 5 Results from analysis of molecular variance (AMOVA) of populations
Phylogenic analysis
Phylogenetic relationships were showed among M. meretrix species and outgroup Meretrix petechialis and Venerupis philippinarum. Because of the limitation in number of M. meretrix samples (3–8 samples per population) and the mixing population among three provines, there was no or less significant differences between populations consensus sequences. Tree topologies indicated that three M. meretrix populations and M. meretrix COI gene sequence (Accession number: DQ399399.1) formed a monophyletic group with very small or no genetic variation among populations.
M. meretrix and M. petechialis have been known as closely related species and there were some suggestions that they should be considered as synonyms4,6. However, as of now, the classification of M. meretrix and M. petechialis are still debated and unanimously agreed on the morphological and molecular biology identification methods. According to previous studies, M. meretrix was only distributed in the South China Sea, while M. petechialis was widely distributed throughout the coasts of China29 and they were often misidentified.20 In another similar case, Chen et al.,4 supposed that M. petechialis and M. lusoria should be treated as a junior synonym of M. meretrix but as the reported by Torii et al.,30 M. petechialis and M. lusoria are the two different species. Moreover, these authors established a method to identify M. lusoria and M. petechialis from shell morphology which can identify with 98.89% correct percentage.
Nineteen out of 59 samples were identified as M. meretrix. The M. meretrix populations had moderate genetic diversity that revealed by values of haplotype diversity and nucleotide diversity. The genetic differentiation (FST) was relatively high, however, the genetic distance (DA) was low and not related with geographic distance. Total genetic variation was intrapopulation variations. There was no clear population structure established among studied populations. The obtained results of this study have contributed scientific basis about genetic data of Asiatic hard clam in some regions in Vietnam. This is the basis for scientists, managers and people to build timely measures to research, preserve and develop clam genetic resources in the future.
The study was supported by Research Institute for Aquaculture No. 1.
None.

©2018 Trang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.