Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 2
1Department of Poultry and Fish Production, Faculty of Agriculture, Menoufia University, Egypt
2School of Environment and Natural Resources, The Ohio State University, USA
3Center for fisheries, Aquaculture and Aquatic Sciences, SIU Southern Illinois University, USA
Correspondence: K Dabrowski, School of Environment and Natural Resources, The Ohio State University, Columbus, OH 43210, USA
Received: July 24, 2023 | Published: August 16, 2023
Citation: Hussein EES, Dabrowski K, Wojno M. Evaluation on the use of free amino acids in diets for red tilapia fries. J Aquac Mar Biol. 2023;12(2):202-207. DOI: 10.15406/jamb.2023.12.00375
An experimental investigation was conducted to address the question of the optimum proportion of protein, free amino acid mixture and test how red tilapia fries will respond to casein-gelatin based diets with partial and/or total replacement with FAA mixture. The protein used was the mixture of casein-gelatin (5:1) and free amino acids matched the casein-gelatin profile. Eight experimental diets based on the use of casein and gelatin (CG)-based diets were analyzed, and five feeding treatments with incomplete replacing of dietary protein with a mixture of FAA with uncertain AA profile. The casein and gelatin were replaced at different levels of 10% (FAA10), 20% (FAA20), 30% (FAA30), 40% (FAA40), and 50% (FAA50) and another two control diets without FAA inclusion. Red tilapia, Oreochromis mossambicus × O. niloticus fries with initial average weight of (0.13 g ±0.01) were fed for an 8-week feeding trial. The results of this study showed that fish fed CG diet had a significant effect (P<0.05) of higher mean final body weight (2.29 g) and specific growth (5.1% /day) than on the Cont 1 diet. During prolonged double feeding, the FAA30 treatment group presented the highest (worst) FCR (1.20) and was significantly different from the cont1 group (P<0.05) gives the lowest (best) value (0.89). The survival rate of the fish fed the experimental diets did not differ significantly with an average of 95.4% (P >0.05).There was no significant difference (P>0.05) in dry matter and ash contents of red tilapia fed with the examined diets. Fish fed the FAA50 and FAA30 diets had the highest lipid content (P<0.05), unlike the other treatments. There was a significant difference in the whole body of red tilapia fed the FAA40 diet (P<0.05), and has a high level of all amino acids compared to FAA30 diet and mostly higher than other groups. Despite the presence of beneficial nutrients such as casein and gelatin mixture, it can be concluded that the FAA components in red tilapia diets can increase growth and feed efficiency.
Keywords: free amino acids, red tilapia, growth performance, nutrition
All cells need a certain amount of amino acids to meet their metabolic needs. The most important concern of animal nutrition is that the absorbability of amino acids must be known so that it can be determined that the food provides satisfactory, but not high, amino acid levels for a generational situation. Fish larvae use amino acids as an energy source, not only for protein synthesis. Many fish species (Tilapia and Oreochromis spp.) are valuable fish products, and understanding their amino acid requirements will assist the research and food industry.1
Nutrient needs are determined in studies with semi-purified diets that comprise well-defined chemical composition and supply outside management over the nutrient under investigation.2 Studies of the composition of free amino acid (FAA) in different organisms showed that free amino acid composition reflects changes in the biological development. In these diets, proteins are partially or totally replaced with a mixture of FAA, giving maximum control over the nutrient under study. Moreover, the potential of fish species to use dietary FAA for growth varies between fish species.
It has been conducted that diets supplemented with FAA are less appropriate for fish growth, compared to the diet based on whole protein.3–5 A higher rate of intestinal absorption compared to amino acids (AA) from whole protein may be the main reason for reduced fish growth causing in different FAA profile in blood plasma and indicates to different assumptions about the destiny of extreme AA in fish blood.6–8
The popularity of hybrid red tilapia due to their similarity to marine species like sea bream (Chrysophrys major) and red snapper (Lutjanus campechanus) with growth performance and feed utilization in freshwater. The popularity is thanks to its market acceptability and for relative tolerance to an oversized range of water quality parameters. However, determining the tilapia stocking density is important to increase its yield, profitability and sustainability.
Therefore, this study investigated how red tilapia responded to the casein-gelatin diet by partially replacing total protein with free amino acids. Also, the aim was to evaluate the effect of adding free amino acids to the diet of hybrid red tilapia (Oreochromis mossambicus × O. niloticus) fries.
Experimental fish
Feeding experiments were carried out with an average small tilapia weight (0.13 g ±0.0) in indoor aquaria at the Fish Research Center of the Faculty of Agriculture, Menoufia University, Egypt. Red tilapia (Oreochromis mossambicus × O. niloticus) was shipped from the government hatchery in Alexandria, Egypt.
Fish were kept ad libitum until the start of the feeding experiment. Fish were randomly divided into groups of 15. Each diet was given to three groups of fish, feeding started at 10% of body weight per day (BW) and gradually reduced to 6% and 4% at the end of the feeding period.2 Feed the fish 3 times at 9:00 and 11:00 a.m. and 14.00 p.m., 7 days a week for two months. Feeding experiments were carried out in glass aquaria (80L) containing dechlorinated municipal water with a constant exchange rate of 0.25L min-1 and water temperature maintained at 26±2 °C. Each aquarium was siphoned twice daily to remove feces. All fish were weighed every three weeks and diets adjusted. Eating is stopped 24 hours before weighing.
All procedures and animal treatment were performed in accordance with Research Ethics and Animal Use (SRE&AUC), Faculty of Agriculture, Menoufia University, Egypt, approval number: 05-SRE and AUC-MUAGR-07-2023.
Experimental diets
Six isonitrogenous and isolipidic testing feeds were utilized in this 56-day feeding test (Table 1). A reference diet dependent on casein and gelatin (CG) and five feeding treatments with incomplete replacing of dietary protein with a blend of FAA with uncertain AA profile. The casein and gelatin were replaced at different levels of 10% (FAA10), 20% (FAA20), 30% (FAA30), 40% (FAA40), and 50% (FAA50) (Table 2). The weight control procedures were set up as indicated by the previous involvement in rainbow trout alevins,9 and common carp hatchlings5 with minor adjustments. The casein and gelatin (5:1) were utilized as the primary protein source and dextrin was replaced with starch in contrast with the first definition. Two "negative" control diets, Control 1 and Control 2, were incorporated which the FAA blend had replaced with starch. Controls are diets without FAA inclusion. The pH of all testing diets was changed in accordance with 7 (±0.1 unit) with 6 N Na OH during the blending of dietary fixings with FAA blends.10,11 So as to diminish FAA draining and increase absorption rate from digestive system, 4% of inorganic commercial binder (Sipernat® 50S, Evonik, Germany) was included. Furthermore, all testing diets were enhanced with 5% of dissolvable fish protein concentrate (CPSP 90, Sopropeche S.A., Boulogne Sur Mer, France) to improve acceptability. Dry fixings were included and blended for stretched out time preceding expansion of oil, water and blended once more. The total blend was handled through 2 mm diameter utilizing the Hobart feed blender (Troy, Ohio) with meat processor connection and the pellets consequently freeze dried. Then the pellets were ground into the appropriate size for the experimental fish.12
|
Ingredients (g/kg) |
Experimental diets |
|||||
|
CG |
FFA10 |
FAA20 |
FAA30 |
FAA40 |
FAA50 |
|
|
Casein |
400 |
360 |
320 |
280 |
240 |
200 |
|
Gelatin |
80 |
72 |
64 |
56 |
48 |
40 |
|
Free AA mixa |
- |
48 |
96 |
144 |
192 |
240 |
|
CPSPb |
50 |
50 |
50 |
50 |
50 |
50 |
|
Starch |
273.4 |
273.4 |
273.4 |
273.4 |
273.4 |
273.4 |
|
Fish oilc |
30 |
30 |
30 |
30 |
30 |
30 |
|
Soy oild |
30 |
30 |
30 |
30 |
30 |
30 |
|
Vitamin mixe |
20 |
20 |
20 |
20 |
20 |
20 |
|
Mineral mixf |
40 |
40 |
40 |
40 |
40 |
40 |
|
NaH2PO4 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Vitamin Cg |
0.6 |
0.6 |
0.6 |
0.6 |
0.6 |
0.6 |
|
Siperant® 50h |
40 |
40 |
40 |
40 |
40 |
40 |
|
Taurine |
15 |
15 |
15 |
15 |
15 |
15 |
|
Choline chloride |
1 |
1 |
1 |
1 |
1 |
1 |
Table 1 Ingredients of the examined diets for red tilapia, Oreochromis mossambicus × O. niloticus fries for 56 days
aConformations (g/240g; all L-form AA otherwise indicated); Arg free base, 9.1; His free base, 3.7; Ile, 4.8; Leu, 7.5; Lys monohydrochloride, 9.6; DL-Met, 5.4; Phe, 9.6; Thr ali o free, 4.3; Trp, 1.1; Val, 6.4; Pro, 59.5; Ser, 59.5; DL-Ala, 59.5
bSoluble fish protein hydrolyzate (Sopropeche S.A., Boulogne Sur Mer, France)
cCod liver oil (MP Biomedicals, Solon, OH, USA)
dSoybean oil (ICN Biomedicals, Aurora, OH, USA)
eVitamin mixture (mg/kg diet) sources were Rovimix series: retinyl acetate, 2.00; cholecalciferol, 0.10; DL-α-tocopheryl acetate, 125.00; menadione niacinamide bisulfite, 5.00; nicotinic acid, 25.00; riboflavin, 20.00; pyridoxine hydrochloride, 15.00; D-calcium pantothenate, 50.00; biotin, 1.00; folic acid, 5.00; cyanocobalamin, 0.05; myo-inositol, 500.00; thiamine mononitrate, 10.00 (Aquaculture Research Group, DSM Nutritional Products France, Animal Nutrition & Health Research, Saint-Louis, France).
fFive milligram Se in the form of sodium selenite per kg Bernhart Tomarelli salt mixture (ICN Pharmaceuticals, Costa Mesa, CA, USA)
gMg-L-ascorbyl-2-phosphate (ShowaDenko K. K., Tokyo, Japan)
hEvonik, Germany
|
AA (g/kg) |
CG |
FAA50 |
|||||
|
Casein* |
Gelatin* |
Sum |
Casein |
Gelatin |
FAA |
Sum |
|
|
Arg |
16.3 |
5.78 |
22.09 |
8.15 |
2.89 |
11.04 |
22.09 |
|
Val |
23.85 |
1.76 |
25.61 |
11.92 |
0.88 |
12.8 |
25.61 |
|
His |
11.45 |
0.61 |
12.06 |
5.72 |
0.31 |
6.03 |
12.06 |
|
Leu |
40.5 |
2.14 |
42.64 |
20.25 |
1.07 |
21.32 |
42.64 |
|
Gly |
7.07 |
18.91 |
25.98 |
3.53 |
9.45 |
12.99 |
25.98 |
|
Ile |
20.68 |
0.9 |
21.59 |
10.34 |
0.45 |
10.79 |
21.59 |
|
Met |
10.84 |
0.57 |
11.41 |
5.42 |
0.29 |
5.71 |
11.41 |
|
Phe |
20.08 |
1.55 |
21.62 |
10.04 |
0.77 |
10.81 |
21.62 |
|
Ala |
16.43 |
6.41 |
22.85 |
8.22 |
3.21 |
11.42 |
22.85 |
|
Tyr |
21.68 |
0.45 |
22.13 |
10.84 |
0.22 |
11.06 |
22.13 |
|
Lys |
33.17 |
3.09 |
36.26 |
16.59 |
1.55 |
18.13 |
36.26 |
|
H-Lys |
0 |
0.69 |
0.69 |
0 |
0.35 |
0.35 |
0.69 |
|
Trp |
5.03 |
1 |
6.03 |
2.51 |
0.5 |
3.01 |
6.03 |
|
Thr |
13.92 |
1.46 |
15.38 |
6.96 |
0.73 |
7.69 |
15.38 |
|
Cys |
0 |
0.07 |
0.07 |
0 |
0.03 |
0.03 |
0.07 |
|
Glu |
70.24 |
7.64 |
77.89 |
35.12 |
3.82 |
38.94 |
77.89 |
|
H-pro |
0 |
8.97 |
8.97 |
0 |
4.49 |
4.49 |
8.97 |
|
Pro |
46.83 |
11.36 |
58.19 |
23.41 |
5.68 |
29.1 |
58.19 |
|
Ser |
13.7 |
2.34 |
16.04 |
6.85 |
1.17 |
8.02 |
16.04 |
|
Asp |
28.23 |
4.29 |
32.51 |
14.11 |
2.14 |
16.26 |
32.51 |
|
SUM |
400 |
80 |
480 |
200 |
40 |
240 |
480 |
Table 2 Amino acid profiles of CG and FAA50 tested diets (examples). Amino acids were provided as protein (casein, gelatin) or in the free form (FAA)
*Source MP Biomedicals, Solon, OH, USA
Diets and whole fish body amino acid analysis
Analysis of the total amino acid composition was performed according to the procedure in accordance with European Commission Directive 98/64 / EC and AOAC method 994.12 and in a validated Evonik (Evonik Industries, Essen, Germany) laboratory. Briefly, samples were hydrolyzed with 6N HCl at 110 °C for 24 hours and neutralized with sodium metabisulfite.13,14 Amino-acid profile analysis of feeds by ion exchange chromatography and post column derivatization with ninhydrin. The presence of AA in the sample was determined by measuring the absorbance of the ninhydrin reaction product at 570nm and adding the value against the internal standard. Tryptophan was measured after alkaline hydrolysis with barium hydroxide octahydrate at 110 °C for 20 hours, followed by fluorescence detection HPLC (extinction 280 nm, emission 356 nm).12
Tumors of three fish in each tank were taken from the back of the body between the dorsal fin and the head. Amino acids were determined by high performance liquid chromatography after acid base digestion by ion exchange chromatography. High performance liquid chromatography (HPLC, Shimadzu Model LC-10AT, Japan) analysis was performed using a manual amino acid analyzer. It is performed according to standard procedure by the commercial analytical laboratory.
Sample pool and examination
Investigations of crude protein, moisture, and ash were performed by standard methods (AOAC 2012). By the end of feeding trial, all fish were counted and weighed to figure percent weight gain which calculated as follows: WG%= [Final bod weight – Initial body weight] × 100/initial BW), feed conversion ratio (FCR; dry feed consumed/WG), Specific growth rate (SGR; 100 × [ln final BW – ln initial BW] /experimental days), and the survival rate = 100 × ([no. of survived fish at the end of the feeding trial /no. of stocked fish at the beginning]).
Besides, six fish from each tank were caught for biochemical examination. Fish were homogenized separately and frozen at -18 °C for proximate examination according to standard methods15 at the research center of the Faculty of Agriculture at Menoufia University, Egypt. Tests were conducted as follows: dry matter, protein, lipid and ash were analyzed after drying in the oven (105 °C for 24h), crude protein (micro Kjeldahl, N x 6.25), crude lipid (ether extraction by soxlhet technique), and ash (600 °C furnace for 5h).
Water quality
Water quality parameters were assessed each other day utilizing a YSI Model 58 oxygen meter. Ammonia and nitrite were estimated utilizing a DREL, 2000 spectrophotometer. Absolute alkalinity and chloride were checked twice week after week utilizing the titration technique; pH was observed twice week after week utilizing an electronic pH meter. During the 8-week feeding trial, the water-quality parameters averaged (±SD): water temperature, 26± 2.2 °C; dissolved oxygen, 6.5 ±0.5 mg -l ; total ammonia, 0.10 ±0.14 mg -l ; nitrite, 0.06 ±0.05 mg - l ; total alkalinity, 181 ±45 mg -l ; chlorides, 575 ±150 mg-l ; pH, 7.9 ±0.4.
Statistical analysis
Each experimental diet was fed three groups of fish at full completion time. Comparisons of treatments were analyzed by oneway analysis of variance. Significance of P<0.05. The differences were tested with Duncan's range test.16
Growth performance
The mean body weight change of red tilapia fed examined diets for 56days is shown in Figure 1. The growth of examined fish is shown in Table 3. Fish fed the CG, Cont1, and FAA10 diets grew faster than fish fed the other diets, resulting in higher body weight than fish fed the Cont2, FAA20, FAA40, FAA50, and FAA30 diet. Fish fed CG, Cont1 and FAA 10 had a higher weight percentage compared to other treatments. The highest values for specific growth rate (SGR%) were observed in fish fed CG, and the lowest values were observed in fish fed FAA 30 diet. Fish fed the FAA30 and FAA50 diets were consumed less than fish fed the other diets (Table 3).During prolonged paired feeding, the FAA30 treatment group presented the highest (worst) FCR (1.20) and was significantly different from the Cont1 group, which yielding the lowest (best) value (0.89) value (P<0.05) (Table 3, Figure 2). The survival rates of fish fed the examined diets did not differ, with an average of 95.4% (Figure 3).
|
Values* |
CG |
FAA10 |
FAA20 |
FAA30 |
FAA40 |
FAA50 |
Cont 1 |
Cont 2 |
|
FBW (g) |
2.29±0.3c |
1.74±0.2ab |
1.61±0.3a |
1.42±0.2a |
1.52±0.2a |
1.51±0.1a |
2.07±0.3bc |
1.64±0.2a |
|
WG (%)† |
1612 ±111c |
1236±127abc |
1118±282ab |
993±298a |
1043±125a |
1105±123ab |
1500±359bc |
1195±131ab |
|
SGR (%)‡ |
5.1±0.2c |
4.6±0.2abc |
4.5±0.4ab |
4.2±0.5a |
4.4±0.2a |
4.4±0.2ab |
4.9±0.4bc |
4.6±0.2abc |
|
FI (g) |
28±4.3b |
24±1.2ab |
23±2.9ab |
21±2.3a |
22±2.5ab |
21±1.2a |
25±3.4ab |
22±5.2ab |
|
FCR§ |
0.96±0.1ab |
0.98±0.1ab |
1.09±0.1ab |
1.20±0.2b |
1.12±0.1ab |
1.14±0.2ab |
0.89±0.1a |
1.17±0.2b |
|
Survival % |
91.1±10.2 |
100±0.0 |
97.8±.3.8 |
93.3±6.7 |
95.6±3.8 |
91.1±10.2 |
97.8±3.8 |
86.7±17.6 |
Table 3 Development of Red tilapia Oreochromis mossambicus × O. niloticus fries (initial wt 0.1 g) and feed utilization after 56 days
*There is no significant difference between numbers with the same letters on the same line (P>0.05). Values are the mean ± SD of triplicates.
†WG (%) = 100 (final body weight – initial body weight) / initial body weight
‡SGR (%/day) = 100 (Ln final weight – Ln initial weight) / Time (days)
§FCR = Total feed consumed (g fish-1) / weight gain (g fish-1)
Body conformation
There was no significant difference (P>0.05) in the body compositions of red tilapia fed examined diets in terms of dry matter and ash (Table 4). The lowest values for ash were found in fish fed the FAA50 diet, followed by fish fed the FAA 10 diet. Fish fed the FAA20 and Cont1 diets showed higher protein content than fish fed the other diets. Fish fed the FAA50 and FAA30 diets had the highest lipid content (P <0.05, Table 4). Levels of all amino acids were higher (P <0.05) in red fish fed the FAA40 diet compared to fish fed the FAA30 diet and generally higher than in fish fed the other diets (Table 5). There was no significant difference in the FAA of red tilapia (aspartic acid, methionine, serine, threonine, valine) between treatments (P>0.05). Fish fed FAA 30 had the lowest levels of alanine compared to the other treatments. The arginine level was highest in the Cont1 group, followed by the FAA40 and Cont 2 groups, and the arginine level was the lowest in the FAA20 group comparing to other groups, the FAA30 group had the lowest leucine levels. However, the FAA10 group had the lowest lysine levels. In addition, the concentration of tyrosine was the highest in FAA40 group but not significantly different than FAA10, Cont1, Cont 2, and FAA50 groups. Moreover, isoleucine level was the lowest in CG group. All other amino acids are listed in Table 5.
|
Items |
Initial |
CG |
FAA10 |
FAA20 |
FAA30 |
FAA40 |
FAA50 |
Cont1 |
Cont 2 |
|
Dry matter |
33.3 |
24±3.0 |
23.9±0.9 |
23.3±1.1 |
22.6±1.6 |
23.5±2.3 |
23.9±4.5 |
23.9±1.5 |
24.2±2.8 |
|
Protein |
56.9 |
48.9±3.7a |
54.0±7.9ab |
63.0±9.1b |
51.9±8.1ab |
50.2±4.2a |
46.0±3.3a |
57.8±6.1ab |
54.7±3.7ab |
|
Lipid |
4.2 |
4.4±0.5a |
4.7±0.2a |
4.9±0.2ab |
6.6±1.1c |
6.1±0.6bc |
7.8±0.1d |
4.7±0.3a |
5.6±1.2abc |
|
Ash |
13.4 |
13.4±0.6 |
12.5±2.1 |
12.7±0.8 |
13.1±1.4 |
13.5±2.4 |
12.3±1.7 |
13.2±3.0 |
14.2±2.0 |
Table 4 The conformation of red tilapia body after feeding with or without FAA for 56 days
Values with the same letters in the same line are not different (P >0.05). Values are mean SD
|
Amino acid (g/100g) |
Experimental diets |
||||||||
|
Initial |
CG |
Cont1 |
Cont2 |
FAA10 |
FAA20 |
FAA30 |
FAA40 |
FAA50 |
|
|
Alanine |
1.6 |
0.41±0.42ab |
0.71±0.37ab |
0.55±0.08ab |
0.35±0.26ab |
0.42±0.41ab |
0.31±0.12a |
0.81±0.38b |
0.53±0.46ab |
|
Arginine |
0.65 |
0.51±0.03bc |
0.77±0.20d |
0.70±0.07cd |
0.42±0.14ab |
0.31±0.01a |
0.46±0.0ab |
0.73±0.08d |
0.57±0.17bcd |
|
Aspartic acid |
0.32 |
0.23±0.14a |
0.43±0.50a |
0.25±0.30a |
0.25±0.30a |
0.11±0.11a |
0.09±0.02a |
0.45±0.48a |
0.29±0.25a |
|
Glutamic acid |
1.69 |
2.93±2.25ab |
3.98±0.46ab |
3.59±2.23ab |
2.4±1.01ab |
1.5±0.79ab |
0.90±0.72a |
4.22±2.16b |
3.52±2.03ab |
|
Glycine |
0 |
0.00a |
0.00a |
0.14±0.06b |
0.00a |
0.00a |
0.00a |
0.00a |
0.00a |
|
Histidine |
0.27 |
0.13±0.06abc |
0.28±0.11de |
0.25±0.06cde |
0.03±0.0ab |
0.07±0.0ab |
0.00a |
0.32±0.16e |
0.16±0.06bcd |
|
Isoleucine |
0.3 |
0.00a |
0.13±0.02c |
0.11±0.03c |
0.11±0.03c |
0.03±0.03ab |
0.03±0.0ab |
0.14±0.05c |
0.08±0.07bc |
|
Leucine |
1.66 |
0.17±0.09ab |
0.54±0.14ab |
0.50±0.40ab |
0.53±0.41ab |
0.12±0.20ab |
0.06±0.06a |
0.58±0.36b |
0.40±0.17ab |
|
Lysine |
1.54 |
0.26±0.03ab |
0.59±0.02c |
0.51±0.29bc |
0.10±0.04a |
0.21±0.0a |
0.32±0.00abc |
0.54±0.22c |
0.34±0.19abc |
|
Methionine |
0.28 |
0.08±0.05a |
0.14±0.16a |
0.09±0.12a |
0.11±0.04a |
0.03±0.0a |
0.00a |
0.14±0.10a |
0.10±0.02a |
|
NH+4 |
1.81 |
0.25±0.06ab |
0.39±0.04bcd |
0.55±0.13d |
0.36±0.0bc |
0.18±0.03a |
0.42±0.00bcd |
0.46±0.20cd |
0.33±0.09abc |
|
Phenylalanine |
2.48 |
0.55±0.30ab |
0.69±0.69ab |
0.63±0.23ab |
0.67±0.29ab |
0.20±0.10ab |
0.00a |
0.82±0.17b |
0.58±0.54ab |
|
Serine |
1.01 |
0.09±0.11a |
0.18±0.14a |
0.16±0.11a |
0.09±0.02a |
0.04±0.01a |
0.03±0.01a |
0.20±0.18a |
0.13±0.09a |
|
Threonine |
0.57 |
0.17±0.23a |
0.31±0.24a |
0.27±0.22a |
0.17±0.12a |
0.09±0.06a |
0.06±0.04a |
0.38±0.45a |
0.22±0.24a |
|
Tyrosine |
0.9 |
0.17±0.15abc |
0.27±0.11bc |
0.26±0.06c |
0.29±0.19bc |
0.17±0.06ab |
0.14±0.02a |
0.36±0.13c |
0.21±0.02abc |
|
Valine |
0.97 |
0.19±0.18a |
0.31±0.21a |
0.26±0.21a |
0.11±0.01a |
0.08±0.10a |
0.03±0.01a |
0.30±0.08a |
0.22±0.24a |
Table 5 Effects of FAA in the experimental diets on amino acids profile of the examined fish
The same superscript letters in the same line are not different (P >0.05). Values are mean SD
The investigation was conducted to address the optimum protein proportion and how red tilapia Oreochromis mossambicus × O. niloticus fries respond to casein -gelatin based diets with different levels of free amino acids replacement. It has been shown that diets based solely or partially on FAA as a source of nitrogen do not meet the maximum potential of fish growth.3,17 The low growth rates of fish especially a gastric fish such as carp were observed with using FAA mixture in the diets.4,5,18,19 This may be due to leak to water before ingestion or during chewing diets in pharyngeal teeth according to Yamada and Yone.20 In addition, FAA solubility in the neutral pH of the intestine may generate high levels of FAA in blood plasma with different profiles associated with the reaction once whole protein-based diets are fed.6–8 That may result in a reduced process of protein synthesis.21 In our study, fish fed the diets CG, Cont1and FAA10 grew faster comparing to other groups (Table 3, Figure 1). Weight gains of fish fed FAA30 diet were the lowest and not different from FAA20, FAA40, FAA50 and Cont2 groups. The FCR was the lowest in the FAA10 group comparing to all other groups except Cont 1 and CG groups (Figure 2). No mortality of the fish was found in FAA10 group compared to all other treatments (Figure 3). The mortality was highest in fish fed Cont 2 diet. The 30% of FAA included in the tested diet resulted in decreasing the fish performance without significant differences (P>0.05). Fish received diets supplemented with 10-20% FAA gained the best growth performance compared to the inclusion 30-50% FAA. Moreover, the 10% of FAA resulted in increased growth rate and decreased FCR. The proper addition level (Cysteine, 10mm) with FAA may be promote gustatory attraction and intake of CG based diet11,22 thus, caused the faster, whole digestion, and the more effective diet utilization. Additionally, the amazing outcomes were gotten subsequent to contrasting FAA30 and FAA50 and the negative controls (Cont 1 and Cont 2, individually). These outcomes recommend the FAA blend was not used proficiently; one of the clarifications being that protein level in our diets was possibly higher than required for red tilapia at this life organize in these trial conditions. The same results were observed with carp.12 It has been shown that juvenile flounder fed diets with reduced levels of amino acid resulted in decreasing growth.23
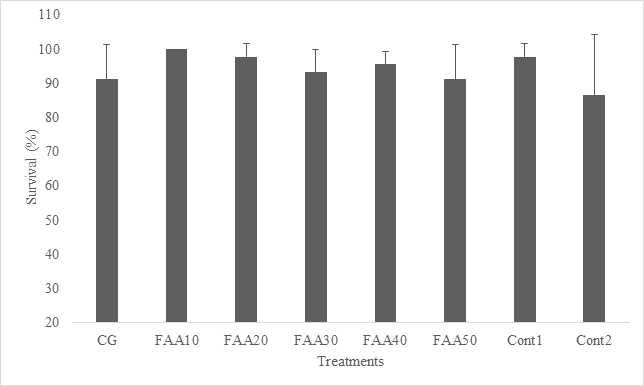
Figure 3 Red tilapia fries performance on semi-purified diets with free amino acids mixture inclusion.
When lupine and pea protein concentrates were fed to rainbow trout (Oncorhynchus mykiss) as part of FM, similar growth and total body amino acid composition were observed across all treatments, despite a 9-12% variation in dietary amino acid composition.24 The amino acid requirement of Nile tilapia has been studied by evaluating casein-gelatin supplements with crystalline Lamino acids, and it is estimated that small Nile tilapia can use up to 82% of the protein in the experimental diet as crystalline amino acids.1
Fish fed 10-20% FAA mixture supplemented diets showed the best FCR (Table 3, Figure 2). This may recommend that this supplementation supports powerful body protein blend in red tilapia, identical to the protein-based diets fed fish with the adjusted essential amino acids. The same results were observed in koi carp with the same diets.12
No significance differences were observed in growth performance and feed efficiency in juvenile Senegalese sole (Solea senegalesis) after 55 days feeding trial where the 50% replacement of whole protein sources (steam dried fishmeal) was replaced with FAA mixture. Furthermore, no effect on the survival was observed by increasing the inclusion level 50-80%.25
Mortality was the least of the feeding trial, with no significant among all treatments, and the lowest rates were observed in those fed Cont 2, followed by the FAA50 and CG diets (Table 3, Figure 3). Except for lipid values, there was no significant difference in total body composition, which was different in all treatments, with the highest values obtained for fish fed FAA50 and the lowest was in FAA 10 group (Table 4). These results are in contrast to Wojno,12 who studied the same diet for koi carp and found no difference between fish fed FAA10 (largest) and FAA40 (slowest growth). In our study, there was no significant difference between the negative control group and CG, FAA10 and FAA20.26
The fish fed FAA50 diet achieved the highest lipid content compared to the other diets. It can be concluded that the FAA mix in red tilapia fries diets improves growth and feed efficiency, despite the presence of high-quality nutrients such as casein and gelatin mix. According to the data, the combination of casein-gelatin and free amino acids can improve growth and feed utilization in red tilapia (Oreochromis mossambicus × O. niloticus) fries.
None.
The Authors declare that there are no conflicts of interest.

©2023 Hussein, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.