Journal of
eISSN: 2378-3184


Review Article Volume 10 Issue 1
1UFR des Sciences Agronomiques, de l’Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Sénégal
2Genome Centre at Max-Planck Institute for plant breeding research, Germany
3Agence Nationale d’Insertion et de Développement Agricole (ANIDA), Sénégal
4Station piscicole de SENAQUA, Tille Bobou, Sénégal
5Agence Nationale de l’Aquaculture (ANA), bureau régionale de Kolda, Sénégal
Correspondence: Mbaye Tine, UFR des Sciences Agronomiques, de l’ Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Route de Ngallèle BP 234, Saint-Louis, Sénégal
Received: January 24, 2021 | Published: February 15, 2021
Citation: Tine M, Ngom A, Senghor S, et al. Enhancing the supply and self-sufficiency of animal protein for local population through artificial propagation of African sharptooth catfish Clarias gariepinus (Burchell, 1822). J Aquac Mar Biol. 2021;10(1):13-24. DOI: 10.15406/jamb.2021.10.00303
The African sharptooth catfish, Clarias gariepinus Burchell, 1822 is the second most farmed fish species in Senegal due to its high fecundity, good growth performance and tolerance of a wide range environmental condition. Despite its economic importance, much remains to be done to improve the production of the species under controlled conditions. The main objective of this study was to implement a suitable method for seed production to meet the increasing demand for fish through the induction of C. gariepinus spawning by hormonal injection using ovaprim and pituitary gland extracts of C. gariepinus. The experiments were independently conducted in three different locations in Senegal: National Aquaculture Agency (ANA) hatchery (Kolda, Kolda), the National Agency for Agricultural Integration and Development (ANIDA) fish farm (Maraye village, Dagana, Saint-Louis) and SENAQUA farm (Tille Bobou, Khombol, Thiès). The results of hormonal induction with ovaprim (dose: 0.5ml/kg of female body weight) stimulated evolution, with 122,500, 150,000 and 15,000 eggs for the ANA, ANIDA and SENAQUA experiments, respectively. The induction with pituitary gland extracts did not, however, provoke female ovulation, probably due to the low dose of hormone contained in these extracts. The hatching rate of the three localities was 60% (73,500 larvae), 30% (45,000 larvae) and 48% (72,000 larvae), respectively. An average growth of 0.25cm/d was recorded for the ANA larvae that were fed exclusively with zooplankton from the third day after hatching, with an alternation of artificial food and zooplankton on the sixth day and with artificial food only on the fourteenth day. However the larvae in this locality had a very high mortality rate of 95%. The mortality rate was also extremely high at ANIDA (96%) where larvae were fed with artemia the second day after hatching throughout the first week, and at SENAQUA farm (98%) where larvae were exclusively fed with dry artificial food (exogenous) from the third day, which corresponds to the resorption of the yolk sac. The high larval mortalities recorded in this study may be due the diet and/or poor water quality in the rearing tanks. These parameters are crucial for the success of larval breeding of C. gariepinus. Based on these results, we suggest increasing by 2 to 3 pituitaries/female and see the effect of this increase on C. gariepinus ovulation.We therefore recommend monitoring breeding conditions after hatching and feeding the larvae with an appropriate diet, preferentially with live food to obtain a better yield, which could help improve production and open other perspectives for the development of aquaculture in Senegal.
Keywords: clarias gariepinus, ovaprim, catfish, fry, natural food
Fishery products are the primary source of animal protein for both urban and rural populations, especially in the poor countries.1-4 The demand for fish protein continues to grow while the production of capture fisheries has been relatively stable since the 1980s.5,6 The state of the fishery resources shows that about 47% of the main stocks or groups of species are fully exploited and have therefore reached maximum limits.7 This overexploitation of fisheries resources, associated with the challenges of feeding more than 9 billion people by 2050, the adverse effects of climate changes and the growing competition for natural resources,8 pose serious issues that need to be addressed. Aquaculture, which comprises the captive breeding of marine, brackish and freshwater fishes, crustaceans, mollusks and seaweeds, constitutes the main alternative that may allow solving this challenge. It has been reported that the worldwide fish supply for consumption has grown faster between 1961 and 2013 than the human population.8 Aquaculture was largely responsible for such an impressive growth in fish supply, with a rate of 7% in 1974, 26% in 1994, 39% in 2004 and 42% in 2014.8 It has become an important sector to fill the gap in fisheries products in all continents.9,10 Aquaculture has grown considerably and globally, and is considered as an integral part of the means used to achieve food security and global economic development.11
Studies have shown that almost the entire Senegalese population consumes fish products daily and fisheries captures are the major source of animal protein in the country.12,13 The great demand for fishery products has resulted to an overexploitation of fisheries resources, leading to their considerable decrease.13 As a result, large species such as Clarias gariepinus, Heterobranchus longifilis, and Heterotis niloticus become more and more scarce and are substituted by small pelagic fish.14,15 The considerable decrease in capture fisheries, while national demand in fishery products increases, has led to an imbalance between supply and demand. The main strategy to compensate for this deficit in fish products is to promote fish farming,which should be considered as a pillar national food security. Aware of the importance of fish farming, the State of Senegal has committed itself to develop the aquaculture sector by setting up the National Aquaculture Agency (ANA), the National Agency for Agricultural Integration and Development (ANIDA), and by opening fisheries and aquaculture training centers. These different agencies and institutions aim at providing employment and economic incomes to rural populations. They are managing several modern fish farms created by the Senegalese government to meet the increased demand for food in general, and fish protein, in particular. The objectives of these farms are to initiate and sustain a stabilizing activity on the rural economy, but also to promote a healthy diet and a dietary diversity in terms of protein for local populations.
The main question that arises is which species should be raised to quickly compensate for this imbalance. One of the most effective answers is the farming of species with high growth potential, such as the African sharptooth catfish Clarias gariepinus.8,15-20 This species isan excellent model for fish farming in tropical and sub-tropical regions because it can grow and reproduce in a wide range of environmental conditions.21-24 Clarias gariepinus is a very hardy species that tolerates stress induced by handling.25-30 The omnivorous diet of C. gariepinus allows the species to eat almost anything, including a variety of cheap artificial foods, which contributes towards its easy breeding.31,32 The species has a very fast growth of up to 5g per day, with a shorter breeding time (3 to 6 months).33,34 Its flesh is highly appreciated by the large majority of the African population, which makes the species commercially valuable.34,35 Its commercial value is even higher when processed.37,38 Among these processed fish products, the fermented, salted and dried fish is the most popular, even in the sub- region from where it is exported to the rest of the country.39,40 Being a freshwater or brackish water fish, C. gariepinus can be raised in freshwater systems, far away from the sea, such as rivers, swamps, lakes, ponds, and streams. The spawners produce large quantities of eggs and sperm throughout the year.21,36,41,42 The species tolerates high stocking densities and large environmental variation in breeding conditions,14,43-45 which makes it the preferred model for aquaculture in tropical countries. All these characteristics make C. gariepinus an excellent candidate for fish farming, particularly in developing countries such as Senegal.
The African sharptooth catfish is the second most farmed fish in Senegal after Tilapia, with an average production estimated around 500kg/year during the period 2001-2003.46 However, the national production of the species is significantly lagging behind the efforts made because of the difficulties in producing of juveniles in captivity. Large-scale farming of this species requires year-round supply of good quality seed fish, which is provided either by collection of larvae from the wild, or through artificial reproduction. While the harvesting of fry from natural sources is quite practical and widely applied in many species, this method has inherent difficulties since it is time consuming, requires considerable skill and experience and is entirely dependent on the productivity of natural spawning grounds.47,48 The collection of C. gariepinus seed from the natural environment for large-scale farming is not the best way because the spawning is seasonal, and fry production is thus required for regular supply to the farmers. Captive breeding is another approach that has attracted attention in recent years and has produced remarkable results. However, there is a considerable research gap in good quality seed production by using induced breeding techniques for acceleration of aquaculture development. Induced breeding techniques described by Viveen et al.49 are being tested locally, and are facilitating the fry supply, allowing thus to improve the aquaculture production. Several methods of spawning induction of C. gariepinus using synthetic steroids (ovaprim) or natural hormones (hypophysation) have been investigated to accelerate the maturation of ovary and spawning.50-59 Although significant efforts are being made to improve the artificial propagation of the species, the adequate conditions for a successful spawning and required experimental facilities constitute a limiting factor in controlling this activity.35,50,59,60 New protocols for spawning induction in C. gariepinus that take into account both the reproductive characteristics of the species and the potentially challenging conditions of the captive breeding locations are thus urgently required.
The present study was carried out mainly to implement a suitable protocol for good seed production to meet the increasing demand for fish products due to increasing population. The experiments were conducted to evaluate the efficacy of pituitary gland extracts and synthetic ovaprim on ovary, spawning, fertilization, hatching rate and viability of larvae of C. gariepinus. They were independently conducted at three breeding facilities located in three different regions of Senegal to take into account the effects of local environmental for the success of the experiments.
Study area
This study was conducted in three different locations: Kolda’s of the National Aquaculture Agency (ANA) hatchery, the National Agency for Agricultural Integration and Development (ANIDA) fish farm and SENAQUA farm, in Senegal. All experiments were conducted during the period from August to October 2017. The ANA agency has several antennas among which the Southern one located in Kolda, which covers the Ziguinchor, Sedhiou and Kolda regions (Figure 1). The ANIDA fish farm is located in the Maraye village of the municipality of Diama, department of Dagana, Saint-Louis region (Figure 1). The SENAQUA farm station is located in TILE BOBOU Khombol, 26km from Thiès region (Figure 1).
Figure 1 Map showing the location of the three different farms in Senegal: the National Aquaculture Agency (ANA) hatchery (Kolda, Kolda), ANIDA fish farm (Maraye village, Dagana, Saint-Louis) and SENAQUA farm (Tille Bobou, Khombol, Thiès).
In the southern zone (Ziguinchor, Sédhiou, Kolda), the climate is warmer, especially during the dry season from February to May (34- 37°C), while in summer (rainy season) the temperatures decrease and are relatively stable around 30/31°C due to increased rainfalls. In the northern zone (Saint-Louis), the climate is hot throughout the year with relatively stable temperatures around 31/32°C except in October and November when they are higher (up to 34°C). The average temperature in the central area (Thiès) is 25.7°C throughout the year. However, in these three areas, temperatures are lower and more variable at night (15-20°C), with cooler winter nights (dry season) and warmer winter nights (rainy season).
In the southern zone, the wet season goes from June to October with an annual average of 1600mm of rain per year. The rainy season extends from July to early October in the northern and central areas and the average rainfall is around 260mm and 503mm of rain per year, respectively. All fish farms in this study are implemented in rural zones where supply of fresh fish from captures remains very low, essentially due to the distance from fishing ports or fish-landing sites. This situation limits the distribution, availability and accessibility of fresh fish products to these rural zones. It is therefore necessary to develop aquaculture production in these rural areas to ensure the supply of fish products to local populations.
The south antenna of the ANA agency has a hatchery located near the dam of Medina Namo in the municipality of Dioulacolon. The hatchery consists of nine (9) concrete ponds with a total capacity of 90m3 or 10m3 per basin, two (2) ponds in liners with a total capacity of 300m3, twelve (12) bins fiberglass including ten (10) circular and two (2) rectangular, floating cages and appas.
This ANIDA fish farm that spreads over 25 hectares has several infrastructures including a hatchery equipped with 12 concrete basins for larval rearing and plastic bins inside and 6 concrete basins outside for broodstock storage, a water tower and a settling basin. The farm is equipped with 44 ponds including: 4 breeding ponds; 20 pre-growing ponds; 20 growth ponds.
The SENAQUA site covers several square meters and is equipped with several livestock infrastructures including 05 concrete ponds of 1m3 designed for rearing C. gariepinus larvae, 10 fiberglass basins of 1.5m3 for rearing juveniles of C. gariepinus, three basins of 96m3, one basin of 60 m3 designed for the enlargement and one hatchery.
Measurements of physico-chemical parameters
The water temperature in the tanks of ANA, ANIDA and SENAQUA experiments was measured using a basic digital thermometer. There was no oximeter and pH-meter to our disposal at the ANA farm to measure dissolved oxygen and pH. Therefore, precautions were taken to maintain water quality. These consisted of filtering the water of the tanks as much as possible, increasing the oxygenation by plugging the filter’s outflow pipe and piercing it on the edges. A protected overflow was also implemented to avoid that the hatched larvae escaped from the emptying well. This continuous water circulation system allowed avoiding the pollution of the water and significant variation of the pH. Similar systems were also implemented for the SENAQUA experiment, which allowed having oxygen saturation but also to haves light variations in water temperature in the tanks throughout the experiment. At the ANIDA farm, pH and dissolved O2 were monitored every day, throughout the duration of the experiment using a multi-parameter analyzer (oximeter, pH-meter). The contents of ammonium (NH4+), nitrite (NO2-), nitrate (NO3-) and dissolved phosphate (PO4-) were also measured in this farm using test kits.
Selection of spawners
The success of artificial production in fish depends on the choice of the spawners, which must be healthy and well fed (Figure 2A). The males and females of C. gariepinus are easily distinguishable during the adult stage on the basis of genital papillae, which are rounded and protruding in the females while males have long pointed genital papillae.61 Mature females typically have a swollen and soft belly which is often full of eggs that are released at the slightest abdominal pressure (Figure 2B). These characteristics are good signs of sexual maturity and were therefore used to choose females for the artificial reproduction. After being selected, females were weighed (Figure 2C) and stored in another tank (Figure 2D) where they were starved to completely empty the digestive system. This allowed avoiding any fecal contamination during the extraction of the eggs, which was done approximately 36 hours later. For males, the largest individuals were selected since they tend to have well-developed testicles, which are full of milky sperm. The selected males were then weighed and kept fasting in the storing tanks. The spawning induction of the broodstock was then carried out by hormonal injection.
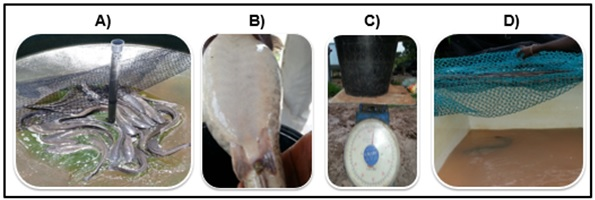
Figure 2 The steps preceding hormonal injection of the female; (A) selection of broodstock, (B) selected female, (C) weighing of selected females, (D) the storage of female in the tank.
Injection of the female
The hormonal induction accelerates fish gonadal maturation during a given period, depending on the nature and concentration of the hormone used, but also on the ambient temperature. In this study, both synthetic ovaprim hormone (Syndel Laboratories Ltd, British Columbia, Canada) and pituitary gland extracts were used to induce gonadal maturation, ovulation and spawning in the African catfish.The synthetic ovaprim hormone was purchased from industry while pituitary extracts were obtained from the pituitary glands extracted from selected individuals of African sharp tooth catfish from the ANIDA farm. The doses of ovaprim and pituitary gland extracts injected to each male and female in each locality are indicated in Table 1.Fish selected for the collection of pituitary glands were healthy and fed with an appropriate diet. After selection, they were sacrificed by decapitation. The head was then cleaned and incised from the mouth to preserve the upper part of the skull which contains the pituitary glands. The head was opened by lateral incision of the bones, and the pituitary glands located in the middle of the cranial box, were then extracted. They were then ground in a mortar and the ground material containing the pituitary extracts was diluted in physiological saline solution. The females chosen for the hormonal treatments were selected on the same day when the pituitary gland extracts were prepared, and weighed using an electronic balance.
Spawners |
Weight (g) |
Injected dose (ml) |
Hormone/Pituitary gland extracts |
ANA_Male1 |
1000 |
10 |
Ovaprim |
ANA_Female1 |
1000 |
10 |
Ovaprim |
ANIDA_Male1 |
1000 |
0.25 |
Ovaprim |
ANIDA_Male2 |
1300 |
0.32 |
Ovaprim |
ANIDA_Female 1 |
1400 |
0.70 |
Ovaprim |
ANIDA_Female 2 |
1200 |
0.60 |
Ovaprim |
ANIDA_Male1 |
900 |
Not injected |
Pituitary gland extracts |
ANIDA_Male2 |
850 |
Not injected |
Pituitary gland extracts |
ANIDA_Female1 |
1000 |
05 |
Pituitary gland extracts |
ANIDA_Female2 |
1000 |
05 |
Pituitary gland extracts |
SENAQUA_Female1 |
1500 |
0.75 |
Ovaprim |
SENEAQUA_Female2 |
1500 |
0.75 |
Ovaprim |
SENAQUA_Male1 |
1200 |
0.30 |
Ovaprim |
SENAQUA_Male2 |
1000 |
0.25 |
Ovaprim |
SENAQUA_Male3 |
800 |
0.20 |
Ovaprim |
Table 1 Dose of the synthetic hormone ovaprim or pituitary gland extracts injected into selected males and females a teach locality
The spawning induction of the ANIDA experiment was done by injection of both synthetic ovaprim hormone for four individuals (2 males and 2 females) and pituitary gland extracts for four other individuals (2 males and 2 females) whereas for ANA and SENEAQUA experiments, only ovaprim was used for all individuals to induce spawning(Table 1). For the ANIDA treatment, two males and two females were injected with ovaprim and two females were treated with pituitary gland extracts (Table 1). Males of this farm were not treated with pituitary gland extracts (Table 1). For the SENAQUA experiment, two females and three males received hormone treatments while for the ANA experiment, only one male and one female were treated (Table 1). The injection of selected individuals was done using a syringe with a needle 3cm in length and 0.6mm in diameter (Figure 3). After aspirating the ovaprim hormone or pituitary gland extracts (Figure 3A), the syringe was vertically oriented to remove any remaining air (Figure 3B), before injecting the hormone into the dorsal muscle, between the lateral line and origin of the dorsal fin.62 The female’s head was covered with a damp cloth before pushing the needle 2 to 2.5cm into the dorsal muscle towards the tail at an angle of about 45° (Figure 3C, D). Once the hormone or the pituitary gland extract was injected, the needle was withdrawn and the muscle was immediately rubbed locally to prevent the hormone rising back into the syringe, and to facilitate its distribution in the muscles (Figure 3E). The following steps including sperm collection from male testicles, egg fertilization and incubation were done immediately because any delay would affect the fertility of the eggs.
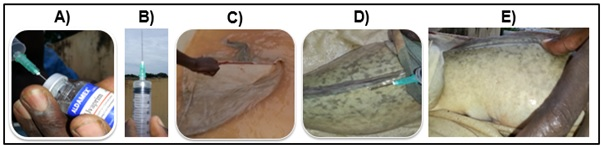
Figure 3 The different steps of hormonal injection: (A) ovaprim aspiration, (B) elimination of the air, (C) capture of the female, (D) injection of the hormone, (E) slight message of the injection area.
Collection of ova and milt and artificial fertilization
Eleven hours after the hormonal injection, the mature females were gently taken from the holding tank, and stripped to collect the ova (Figure 4A, B), which were stored in a small container (Figure 4C). The total number of eggs obtained from each female after stripping was estimated based on the Viveen et al. statement that stipule 200g of eggs correspond to 140,000 eggs (i.e. 1g of eggs corresponds to 700 eggs). Thus, the amount of eggs was obtained for each female by multiplying its egg weight (in g) by 700. Before stripping the females, the selected males (Figure 5A) were sacrificed to collect the testes. After being killed by decapitation, they were placed on the back and the abdominal cavities were opened (Figure 5B). The intestines were cleared to better identify the testicles that are normally located on either side of the spine. The testes were then detached from the dorsal part of the abdominal cavity while avoiding crushing them (Figure 5C). The milt was collected by pressing the sides of the testes, and poured onto the ova (Figure 6A). A 32ml volume of physiological solution was first poured onto the sperm-ovum liquid, and mixed with a bird feather for 2 minutes. Then, a 357ml volume of water was added, and the final solution was mixed with a bird feather to facilitate the fertilization of ova by the spermatozoa (Figure 6B). The total mixture was kept in a bowl for two minutes while mixing its contents (Figure 6C). During this time, the fertilized eggs develop a small adhesive disc on their surface which adheres easily in a few seconds to any support by simple contact. The mixture was rinsed three times to remove any egg debris that may be a source of pollution. The fertilized eggs were then transferred to tassels made of synthetic fibers to promote their adhesion to the supports. The eggs glued to the supports were then placed into trays containing clean water for the incubation and hatching phases.
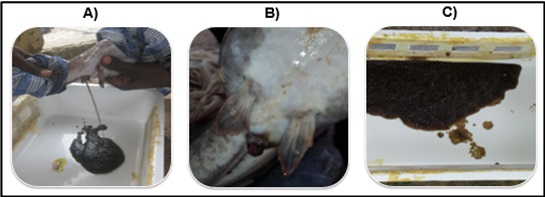
Figure 4 The different steps of egg striping from female; (A) extraction of eggs, (B) egg clutch after extraction, (C) eggs stocked in a container
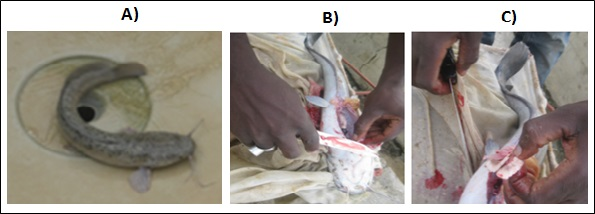
Figure 5 Testicle extraction of selected males; (A) selected male; (B) male dissection; (C) extraction of the testicles.

Figure 6 The stages of artificial fertilization; (A) incision of the testicles, (B) addition of water, (C) fertilization of eggs.
Egg incubation and hatching
The fertilized eggs were incubated in the incubation trays (Figure 7A) using a screened mesh frame with 1mm mesh size. This frame was installed at a depth of 10cm in the tank containing well aerated and dripping water, with a flow of 1to 3l/minute. The eggs were spilled gently in a dispersed manner onto the surface of the frame (Figure 7A, B). The incubation medium was enriched with dissolved oxygen either contained in a gas cylinder or supplied by an air compressor, which allowed the eggs to be appropriately oxygenated (Figure 7C, D). The hatchery exits were closed and the tanks covered with a tarpaulin to minimize variations of water temperature. After a certain period of incubation, the fertilized eggs hatched. The hatching rate (HR) was calculated based on the following formula:
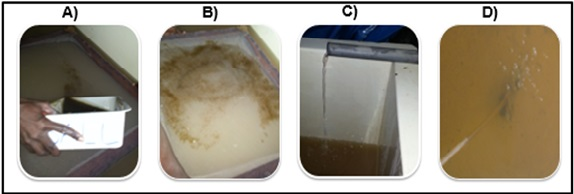
Figure 7 Conditions of the incubation medium; (A) egg spillage, (B) dispersed eggs, (C) inlet water flow, (D) oxygenation with a gas cylinder.
Larval rearing
To avoid high mortalities during the larvae rearing phase, the dissolved oxygen supply in the culture tanks was optimized either with oxygen gas cylinder or oxygen gas compressor. The larvae were fed with natural and/or artificial food. Live food consisted of artemia hatched in the aquaculture facility, Artemia salina (SENAQUA) or native zooplankton collected in nearby ponds (ANA), whereas the artificial food was either locally made (ANA) or purchased from the local market (ANIDA). The zooplankton containing a great diversity of living organisms was collected in ponds using a 100micron mesh dip net (Figure 8A). It was then filtered with a landing net made from mosquito net (Figure 8B) in order to retain all aquatic organisms that are food resources for the feeding of the larvae (Figure 8C).The artificial food was prepared by mixing cassava flour with water and then boiling the mixture until forming a sticky paste (Figure 8D). This paste is mixed with fishmeal and egg yolk to make pellets (Figure 8E).
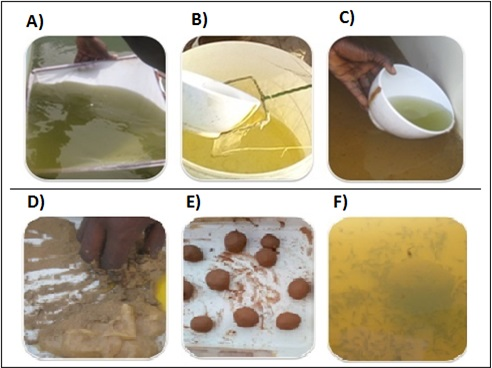
Figure 8 The stages of feeding with live food (zooplankton) and artificial food; (A) Fishing for zooplankton, (B) elimination of undesirable individuals, (C) feeding of the larvae; (D) mixture of ingredients, (E) formed pellets, (F) larvae feeding.
The feeding with zooplankton (ANA farm)started on the third post- hatching in the morning and was done twice a day, in the morning and late in the afternoon (Figure 8C). The larvae were then weaned using the locally made artificial food on the sixth day after hatching, which corresponds to the third day after the first intake of live food. The feeding was done by depositing pellets at the bottom of the larval breeding tank (Figure 8). One hour later, the zooplankton was again distributed to the larvae (Figure 8C) but the tank was siphoned off before each feeding in order to remove any contaminating objects (dead larvae, uneaten food and others). The water supply system was stopped during the siphoning operation to avoid mixing of the water, which could promote a drop in the dissolved oxygen levels in the tank, and then restarted afterwards.
The feeding in the ANIDA farm started with artemia the second day after hatching throughout the first week. The food was distributed by hand, ad libitum, in 4 daily meals (at 9a.m., 12p.m., 4p.m. and 6p.m.) for the duration of the experiment. On the seventh day after hatching, weaning was started by mixing purchased artificial food with the artemia, while gradually decreasing the amount of artemia until day nine. From the tenth until the fifteenth day, artificial food was exclusively distributed. The water in the tanks was renewed at least up to 50% the second day after hatching before feeding, to ensure a good water quality. Additionally, a daily siphoning of the tanks was carried out throughout the duration of the larval rearing.
For the experiments conducted at SENAQUA, the larvae were exclusively fed with dry artificial food (exogenous) from the third day, which corresponds to the time of yolk sac resorption. The feeding ratio was 16mg/individual and was provided in 4 meals a day (at 9 a.m., 12 p.m., 4 p.m. and 6 p.m.). The average sizes of the larvae at the 7th and 21st day (D7 and D21), average size gain and average daily growth were calculated using the following formulas:
The Student’s test.t was used to verify the significance of differences between means of the above parameters. All analyses were completed using the ‘ADE4’ library in ‘R’ software. The significance level is set to 5%.
Physico-chemical parameters
The temperature of the water in the tanks of the ANIDA experiment did not vary significantly (Student’s test.t; p>0.05). Throughout the experimental period, the lowest temperature (28°C) was recorded in the morning and the highest (35°C) in the afternoon. The average daily temperature varied around 29.64±0.55°C.The average dissolved oxygen concentrations varied between 4.4±3.20 and 5.2±0.005mg/l. These concentrations are lower than those of the dissolved oxygen saturation, which is 8 mg/l at 25°C, but they are still adequate for the needs of eggs and larvae. The average pH in the tanks varied between 7.3 and 8. These values are also within the pH range (6.5 to 8) favorable for fish growth. The average contents of ammonium (NH4+), nitrite (NO2-), nitrate (NO3-) and dissolved phosphate (PO4-) obtained at the ANIDA farm were 0.20, 0.03, 2.00 and 0.30, respectively.
For the ANA experiment, the average water temperature recorded at the interval of 2 hours for three successive days did not vary significantly (Student’s test.t; p> 0.05). The water temperature of female storage tanks varied between 29 and 29.5°C (average: 29.25±0.27) whereas that of the incubation tank ranged from 27.5 to 34.5°C, with an average of 30.75±2.46. The water temperature of the larval rearing tanks varied between 28 and 32°C with an average of 29.58±1.57.
Spawning performance
The amount of eggs released by each female treated either with synthetic hormone ovaprim or pituitary gland extracts are indicated in Table 2. For the ANA experiment, the female had already started releasing eggs when it was captured for the striping. This shows that the female reacted well to the hormonal injection, as it is evident from the striping operation that sent out jets of greenish ova, which is a sign of maturity and good success of the hormonal induction on inducing gonadal maturation. The stripping of the female resulted to a spawning weight of 175g, corresponding approximately to a total number of 122,500 eggs. For the ANIDA experiment, the striping of females that received an injection of pituitary gland extracts of C. gariepinus did not release eggs. By contrast, females that were held in the same hatchery under similar conditions, but treated with the synthetic ovaprim, had a higher spawning weight (250g each, corresponding to a total of ~150,000 eggs) after stripping (Table 2). This indicates that females responded favorably to the injection of the synthetic hormone ovaprim. For the SENAQUA experiment, the stripping of females released also a large amount of eggs (total: 150,000 eggs) (Table 2), which shows that they did react very favorably to the ovaprim injection.
N° female |
Type of treatment |
Egg weight (in g) |
Number of eggs per female |
Total number of eggs |
F1_ANA |
Ovaprim |
175 |
122,500 |
122,500 |
F1_ANIDA |
Ovaprim |
250 |
75,000 |
150,000 |
F2_ANIDA |
Ovaprim |
250 |
75,000 |
|
F1_SENAQUA |
Pituitary Gland Extracts |
0 |
0 |
0 |
F2_SENAQUA |
Pituitary Gland Extracts |
0 |
0 |
0 |
F1_SENAQUA |
Ovaprim |
20 |
75,000 |
150,000 |
F2_SENAQUA |
Ovaprim |
18 |
75,000 |
Table 2 Spawning performances of females treated with ovaprim and pituitary gland extracts
For the ANA farm experiment, the first larvae were observed after approximately 22 hours of incubation at an average water temperature of 30.75°C. The hatching rate was 60%, which corresponds to a total of ~73,500 larvae (Table 3).
Farm |
Total number of eggs |
Total number of larvae |
Hatching rate |
ANA |
122,500 |
73,500 |
60% |
ANIDA |
150,000 |
45,000 |
30% |
SENAQUA |
150,000 |
72,750 |
48% |
Table 3 Hatched larvae and hatching rates
The first larvae stages of the ANIDA experiment appeared slightly green and correspond to fertile eggs that have developed flagella which allow them to move continuously. At this stage, the larvae are not yet completely released. There were also white colored eggs that were inert and development of a frothy layer, which is caused by the rotting of unhatched eggs. The number of the hatched larvae was 45,000, which corresponds to hatching rate of 30% (Table 3).
For the experiment conducted at SENAQUA farm, a hatching rate of 48% was recorded, corresponding to a total number of 72,000 larvae (Table 3). The hatching in this farm started 24 hours after incubation in nursery ponds at a temperature of 29°C. For the SENAQUA experiment, the average water temperature showed slight variations (29-30°C).
Larval breeding
The behavior of the larvae was similar before and after feeding. Before feeding, the larvae were less active, almost stable, dispersed in a heterogeneous manner with a low density on a well-determined place of the tank. Right after feeding on this same place, a real migration towards this environment occurred and the larvae became more active (Figure 8C, F). The behavior of the larvae when they received the natural food (zooplankton) was the same as when the artificial food was provided. The migration of the larvae shows a real zooplankton hunting movement (Figure 8C), indicating that larvae feed the natural food. For artificial food, once given a high density of larvae around the dumpling was observed after a few minutes (Figure 8F). Therefore, whichever food was provided (natural or artificial), the larvae are able to detect it.
Comparisons of the larval quality seven days post-hatching revealed that there were poorly-formed and well-formed-larvae (Figure 9A). After a few seconds out of water, the malformed larvae (Figure 9A;2) were dead while the well-formed ones (Figure 9A;1) survived and were active in the water. The average sizes of 7 and 21 day old individuals of the ANA experiment, their average daily growth (in cm) and mortality rate (%) are indicated in Table 4 and Figure 9B, The sizes of the three individuals measured on the 7thday were1.10, 1.20 and 1.40cm, respectively, with an average of 1.23cm (Figure 9B, Table 4). On the 21stday, the size of the same individuals was 4.50, 4.70 and 5.00cm, respectively (Figure 9C, Table 4). The average size of these individuals on the 21st day was 4.73cm. The average size gain was thus 3.5cm, with a daily growth of 0.25cm. After five weeks of breeding, the number of larvae remaining of the 73,500 that originally hatched was 3,500. This corresponds to a mortality rate of 95% (Table 5).
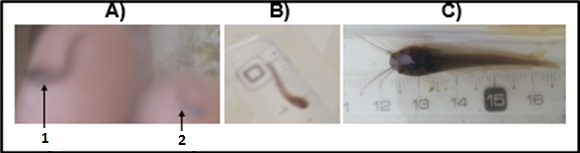
Figure 9 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Growth parameters |
Individual 1 |
Individual 2 |
Individual 3 |
Average size |
Average weight gain (g)
|
Average daily growth (cm) |
Size D7 (cm) |
1.10 |
1.20 |
1.30 |
1.23 |
3.50 |
0.25 |
SizeD21 (cm) |
4.50 |
4.70 |
5.00 |
4.73 |
Table 4 Average sizes of larvae at 7 day (D7) and 21 day (D21) post-hatching, average height gain and average daily growth
Farm or experiment |
Initial number of larvae |
Remaining larvae after 3weeks |
Mortality rate |
ANA |
73,500 |
3,500 |
~95% |
ANIDA |
6,750 |
130 |
~96% |
SENAQUA |
72,000 |
288 |
~98% |
Table 5 Larval breeding and mortality rate
Upon hatching, the larvae of the ANIDA experiment measured 5 to 7mm and weighed approximately 1.2 to 3.0 mg. The yolk sac was absorbed after three days post-hatching and the larvae that become fry started search for food. After a few weeks of rearing, in the fry stage we obtained a number of 130 fry of 30g including a mortality rate of ~96%.
For the SENAQUA farm at least half of the larvae survived until the fifteenth day after fertilization but most of them died after. The total mortality rate in this experiment is ~98%. During this rearing phase, the size and weight of the larvae were not measured, but there was slight growth.
The water temperature is a very important parameter for artificial propagation in fish because it allows estimating the time when the eggs are extracted from the hormone-injected female and the time required to incubate the eggs. Therefore, the water temperature of both incubators and rearing tanks was recorded in this study during the storage of the females, the incubation of eggs and the larval breeding. The results indicate that for the ANA experiment, the average water temperature during storage of the injected female was 29°C, which theoretically corresponds to a latency time (time between injection and collection of C. gariepinus eggs depending on the water temperature) of 7h 30 min.63 The water temperature of the ANIDA experiment varied between 29.35 and 30.55°C, which is in accordance to the range of temperature (26 and 30°C) reported as being optimal for the growth of C. gariepinus.64 Likewise, the water temperature of the SENAQUA experiment, which varied between 29 and 30°C throughout the period of the study, was within this temperature range. However in this study, the time between the hormone injection and the collection of ova for the SENAQUA experiment was 11h, which corresponds to an extra latency of 3h 30mn.This longer period of incubation could lead to the loss of a certain amount of ova by the incubating females. It is, therefore, crucial to respect the time (depending on the water temperature) that must separate the hormonal treatment and the collection of ova in order to optimize the results of the induced reproduction of C. gariepinus.63 The estimated time between egg fertilization and the hatching for a water temperature of 30°C is 20h while, in this study, it was 22h for an average temperature water of 30.75°C. This gives a 2h delay in hatching, which could be due to the slight variation of the water temperature (30.75°C instead of 30°C) in the incubation medium. Although temperature is not a limiting factor for the expression of the reproductive potential of C. gariepinus during the various artificial reproduction assays carried out in this study, the results show that variations in this parameter must be taken into account to respect the latency and therefore avoid egg loss and delayed hatching.
Dissolved oxygen concentrations in the holding tanks of the ANIDA farm ranged from 3.35 to 6.6mg/l. The lowest dissolved oxygen level was recorded in the evening of the second day of the experiment, which was probably due to a dysfunction of the oxygen supply system. The dissolved oxygen values recorded during the various artificial propagation assays were on average greater than 3mg/l and are in the dissolved oxygen range reported by Viveen et al. 49 as being favorable and optimal for an optimal growth of C. gariepinus fingerlings. Dissolved oxygen may have had an effect during testing, as the lowest level coincides with the embryonic stage of development, which may be related to the low hatching rate of 30%.
The pH values recorded during the experiment are between 7 and 7.9 and are within the pH range (6.5 to 9) of optimal growth limits reported for C. gariepinus.65 Furthermore, C. gariepinus has a great adaptation capability even to very poor environmental conditions,66 suggesting that the pH levels recorded in this study did not impact reproduction success.
Many works have been done on the artificial reproduction of C. gariepinus.50-58 However, to our knowledge, this is the first study conducted in Senegal that deals with the spawning induction of C. gariepinus. The results show that it is possible to obtain reproduction and egg-laying by adults of this species with the use of induction hormones. However, the results obtained were very irregular, and the percentage of hatching varies considerably depending on the locality. These amounts of released eggs by females in this study are lower than those reported by Viveen et al.49 who stipulate that the laying weight of C. gariepinus in African hatcheries is around 10% of the females’ live weight of (500g) or 20% of the live weight of females. This difference could be explained by the insufficient of inoculated dose, the diet which is poor in essential amino acids and energy, as well as handling stress, which caused micro-injuries and petechiae which could be factors inhibiting ovulation.29
The reproductive failure of the spawners treated with pituitary gland extracts of the African sharptooth catfish may be related to the low dose of hormones they contain. It has been reported that if the individual sacrificed for the pituitary gland extraction has a smaller or equal size to that of the female to be injected, two pituitaries should be used per female injected. On the other hand, if the size of the donor is more than twice the size of the recipient, a single pituitary gland is enough to induce the maturation and the spawning of the catfish female.48 In the ANIDA experiment, the size of the pituitary donors and the recipients was not significantly different. Therefore, only one pituitary gland was used per female to induce the gonad maturation and ovulation. This indeterminacy of the dose of pituitary extract injected into the spawners could prevent the stimulation of gonad maturation, which could end the ovogenesis and folliculogenesis processes, and therefore lead to the absence of ovulation. This absence of induction of females treated with pituitary gland extracts may also be due to handling stress, which can inhibit egg laying, to the poor quality of hormonal inducers (poor preparation), the poor environmental conditions (physico-chemical parameters) or the use of females whose the vitellogenesis was incomplete. Unfortunately, with the dataset generated in this study, it is difficult to determine the cause responsible for the reproductive failure. Further studies are required to clarify which of these factors might be responsible.
The results of the ovaprim injection recorded in this study indicate that the quantity of ova released varied considerably, and was sometimes less than that obtained usually. The hatching rate was low and (1/3 of the eggs have not hatched), in accordance with Micha7 findings (possibility to have 0 to 70% hatching for a single female). This low hatching rate can be explained by the lack of premarital aggressiveness which occurs during the courtship, a process which is essential in the natural reproduction of C. gariepinus. Indeed, this step allows the female to lay her eggs in small clutches, and her partner to fertilize each clutch at the same time by dropping a cloud of milt above the eggs, hence the fertilization of the majority of eggs. On the other hand, if there is no significant aggressiveness and mobility, the majority of the ova remain unfertilized by the male.
Food plays a major role in the growth of larvae after the reabsorption of the yolk reserve. The migration of the larvae of the ANA experiment shows a real zooplankton hunting movement, which indicate that larvae have a preference for live food. For the artificial food locally made, once given a high density of larvae was seen around each dumpling after a few minutes. The tendency to abandon artificial food for natural food was apparent and could be explained by the fact that the dumplings (artificial food) lasted in water, which caused the loss of their nutritional quality as long as they remain in the water. Given that C. gariepinus is a predatory species, the instinct of predation of the larvae led to hunt the prey (zooplankton).
Although the artificial reproduction with ovaprim was successful, high juvenile mortalities were recorded. Several physico-chemical factors including dissolved oxygen levels can be responsible for these high mortalities. Indeed, the oxygen content of the water plays a crucial role in the success of fish farming, especially during the early developmental stages. The primary source of dissolved oxygen in fish culture tanks is from the phytoplankton. The tanks can be also enriched with dissolved oxygen by the recirculation of the water in the rearing tanks. The high mortality recorded after hatching and during the larval breeding may be explained by the drop of dissolved oxygen levels in the culture tanks. These mortalities, which essentially occurred during the night, are probably not due to a dysfunction of the water circulation system but to an interruption of the photosynthesis. Indeed, before siphoning the tanks in the morning in presence of sunlight, the water circulation system was interrupted throughout the day to optimize the cleaning operation and to prevent losing the zooplankton that remained in the tanks after feeding, but there was no larval mortality during this period.
Most of the larvae could also die during the metamorphosis because it was not complete and consequently the accessory respiratory organ that allows them to rise to the water surface and remove oxygen from the air was not well developed. Indeed, it is commonly known that larvae of C. gariepinus that are more than 12-14 days old have their accessory breathing organ completely developed and can therefore remove the oxygen from air for respiration.
The massive mortality of the SENAQUA farm is probably caused by the poor water quality. Indeed, with the high density of eggs in this tank, the rotting of unhatched eggs causes the development of fungi, leading to a drop in water quality. This is in agreement with the finding that the rotting of unfertilized eggs can cause the pollution of the water in the rearing tanks. It is, therefore, important to frequently check for white eggs in the incubators to preserve the water quality, which unfortunately was not done in this study. The high mortality of about ~96% recorded during larval rearing of the ANIDA farm may be due to the artificial dry food. In fact, two days after hatching, the larvae clearly prefer live food such as artemia hatched in the aquaculture facility or zooplankton collected from the nearby tanks but the larvae of this farm were only fed artificial dry food. Therefore, the larvae may either use this artificial food but it will take time before they get used to it, or they simply did not appreciate it.
In summary, upon completion of works at the ANA, ANIDA and SENAQUA farms, it appears that the mastery of proper techniques of artificial reproduction should allow the rapid development of the reproduction of the African catfish especially in rural areas. The mastery of such breeding techniques will allow seed production and supply, and therefore accelerate the development of fish farming of this economically important species. The acquisition of technical knowledge in the field of seed production is a fundamental condition for the smooth running of all aquaculture activities, including research and production. Indeed, technical proficiency of artificial reproduction of C. gariepinus is one of the solutions to increase local production, to give an opportunity to fish farmers who do not have the appropriate equipment or those who do not have a sufficient quantity of parents intended to be sacrificed to harvest. It plays a key role in the extension and maintenance of the breeding of this species in Senegal. However, further research remains essential in order to better understand the effects of water quality and the exact induction doses, in particular the doses of ovaprim hormones on the success of artificial propagations. Although the artificial reproduction with ovaprim was successfully achieved with satisfactory results regarding the spawning performances (large amounts of eggs produced per female) and hatching rates recorded, there were considerable larval losses. These high mortalities can be explained by the poor water quality due to the absence of the recirculating system and the strict control of physico- chemical parameters such as temperature, dissolved oxygen levels, pH, nitrite and nitrate content. The use of inadequate food to feed the larvae could also be a potential cause of these high larval mortalities. Further investigations on optimal larval rearing conditions in C. gariepinus are needed to overcome the low survival of larvae, which is one of them.
Artificial reproduction can play an important role in achieving the government’s objective of ensuring food security, which relies on aquaculture for its economic development and which wishes to increase the supply and self-sufficiency of animal protein for its population. In view of the results of this study, we suggest to fish farmers who want to embark on artificial reproduction of C. gariepinus to use domesticated healthy broodstock to have good quality and sufficient quantities of gametes and to have at their disposal a well-fertilized pond or pond that will constitute the culture medium for zooplankton (the appropriate food of the first larval stages). In term of perspectives, there are many prospects for developing C. gariepinus production in Senegal. Indeed, given the scarcity of the fish captures on the market, fish farmers must invest in the production of this species which has a future for the development of aquaculture. Several complementary lines of study emerge including the setup a modern hatchery to ensure fry supply, and the local production of live and artificial foods. Water quality measurement equipment is required, including an oximeter to measure the DO at the bottom of the pond plus a complete analysis kit with additional kits for ammonia, nitrites and pH. Another requirement to improve aquaculture local production is to extend the study to other species of catfish of economic interest and finally to deepen the study of food at each stage of life of the species for a good growth.
The induction hormones used have given negative results for the C. gariepinus pituitary gland extracts and positive results for ovaprim, but this does not allow us to conclude the letter is the most effective. Additional research remains essential to determine the exact induction doses, in particular the amounts of pituitary gland extracts needed for successful spawning induction. The results from such studies could allow to realize artificial reproduction of the C. gariepinus by simply using local resources, which could limit the high expenses for purchase of synthetic hormones.
This project has been financially supported by the Senegalese National Aquaculture Agency (ANA), ANIDA and SENAQUA farm. Financial supports was also obtained from the Max Planck Institute for Molecular Genetics (Berlin, Germany) and the University of Gaston Berger (Senegal) for manuscript preparation.
I thank ANA, ANIDA and SENAQUA for offering facilities and helping to conduct the breeding and larval rearing experiments. I am also grateful to Dr Peter Teske for helping for the English editing and for improving the manuscript.
MT, AN, PD and CABF have conceived and planed the study. SS, SFN and BD have conducted the experiments with the help of CABF. MT performed statistical analyses, planned and coordinated the manuscript preparation, and wrote the manuscript. All authors have contributed to the manuscript preparation. They all read and approved the manuscript.
The author declares that there is no conflicts of interest.

©2021 Tine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.